Since their launch in the mid-1980s, therapeutic monoclonal antibodies (mAb) have provided useful tools in treatment of a wide range of diseases, including diabetes, rheumatoid arthritis, and hemophilia. However, most antibodies evolved from non-human species (e.g. mice) were shown to have limited use as therapeutic agents due to an inability to trigger human effector functions and a short therapeutic half-life as they were recognized by the patient' immune systems as foreign proteins resulting in a human anti-mouse antibody (HAMA) response. In the contract, human mAbs possess diverse advantages over animal-derived antibodies, thus attract increasing attention for clinical applications, such as prevention or treatment of microbial infection, immunotherapy of toxins and diagnosis by antibody-targeted radioisotope imaging.
The process of "humanization" is usually applied to monoclonal antibodies that are developed for administration to humans but are generated in a non-human immune system, as the protein sequences need to be modified to increase their similarity to antibody variants produced naturally in humans. To date, there are quite a few antibody engineering techniques employed for antibody humanization to reduce immunogenicity and the HAMA response, such as CDR grafting, phage display, human hybridoma and approaches using transgenic mice.
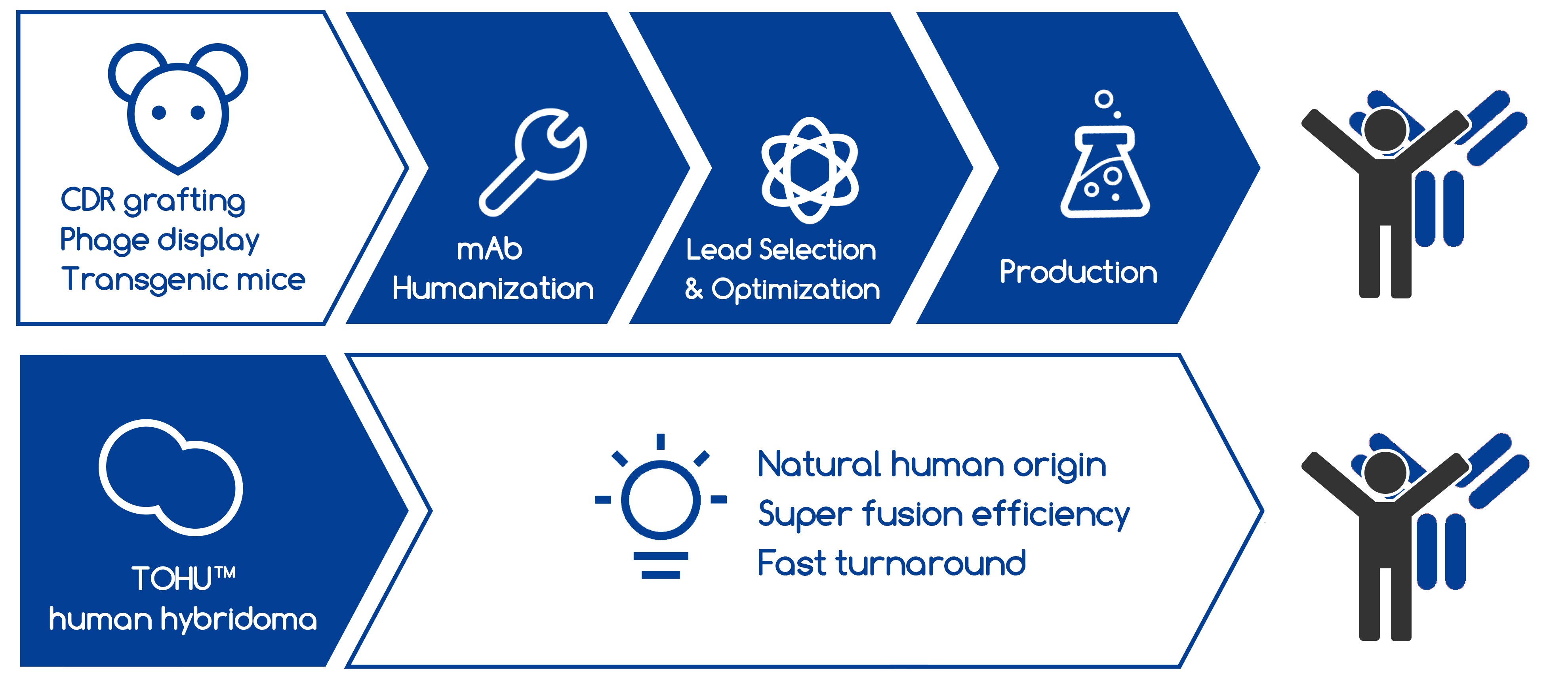
Among these humanization strategies, hybridoma technology has several appealing features. Human hybridoma technology uses human cultured cells as a fusion partner, allowing the generation of natural human antibodies in a native form, which is crucial for Fc-mediated activities. Human hybridoma technology is the most direct way for humanized antibody development, as it requires no additional modifications for production of therapeutic antibodies. In addition, it has several appealing features, including a relatively simple protocol, low costs, and the ability to immortalize antibody-producing cells.
Creative Biolabs fully understood the difficulty in the preparation of hybridomas by human-human crosses. Today, our in-house scientists are proud to provide the newly developed TOHU™ human hybridoma platform to overcome limitations existing in current hybridoma technologies, such as low efficiency in fusion and production, reduced binding affinity to target, impairment of desired immune response, potential for unfavorable immune response, and rapid intrinsic clearance.
 One-on-one
One-on-one Nearly 100%
Nearly 100%  Very few cells
Very few cells Fast turnaround without
Fast turnaround without This cutting-edge technology constitutes a highly efficient and deep mining process and provides an unprecedented capability to produce totally human antibodies with high affinity and functional activity. This unique capability of TOHU™ platform represents a major advantage compared to other technologies and enables the development and manufacture of unique therapeutic antibodies.
Our TOHU™ human hybridomas platform has been designed to develop antibodies for research purposes only. Do not use them for clinical or therapeutic applications.