ADC Development Services for Non-Small Cell Lung Cancer (NSCLC) Research
The introduction of targeted therapies and checkpoint inhibitors for non-small cell lung cancer (NSCLC) in the past decade has improved the treatment landscape for patients with advanced or metastatic NSCLC. For those who are not eligible for current treatments or eventually develop resistance to the drugs, new therapeutic approaches are needed. Nowadays, NSCLC patients treated with antibody-drug conjugates (ADCs) have improved progression-free survival and overall survival, highlighting the benefit of precision medicine. Armed with extensive working experience in antibody design and bio-conjugation, Creative Biolabs is dedicated to helping our clients design customized ADCs using our well-established technology platforms. We offer comprehensive Antibody Screening Services, "Warhead" Assembling Services and intact ADC Preparation Services. We demonstrate that the resulting ADCs are promising candidates for scientific and preclinical research against NSCLC research.
Introduction of NSCLC
NSCLC accounts for ~85% of all lung cancer. Tobacco smoking remains the main risk factor for developing NSCLC, but radon exposure and air pollution also have a role. Many patients are diagnosed with advanced-stage disease owing to inadequate screening programs and late onset of clinical symptoms, consequently, patients have a very poor prognosis. There are different subtypes of NSCLC that are grouped together because of the similar treatment as well as prognosis approaches. These subtypes are squamous cell carcinoma, adenocarcinoma, large cell carcinoma and more poorly differentiated variants.
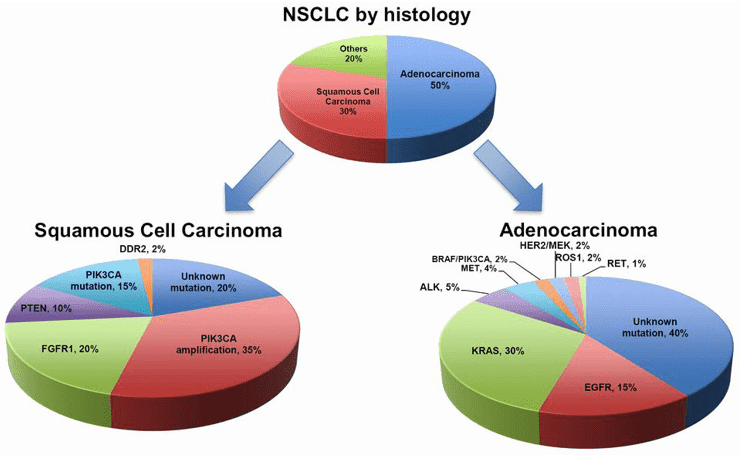 Fig.1 NSCLC by histology and mutations. (Chan, 2015)
Fig.1 NSCLC by histology and mutations. (Chan, 2015)
Treatment of NSCLC
With the advent of genetic and molecular techniques to characterize the driver mutations at the cellular level, treatment options for NSCLC have evolved tremendously over the past years. Treatment modalities are being used including traditional surgery, radiation therapy, chemotherapy, laser therapy and newly emerging targeted therapy, as well as immunotherapy and combination therapies. Among these treatments, traditional chemotherapy has nonspecific toxicity and a narrow therapeutic window. Targeted therapies such as epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) can provide safer and more effective treatments, but drug resistance is the new challenge to targeted therapies. Besides, less than one-third of NSCLC patients expressing activated EGFR respond to TKIs and don’t significantly benefit from TKIs compared with patients in chemotherapy. Furthermore, the outcomes of an anti-EGFR antibody, cetuximab, in a multi-center clinical trial have shown no significant increase in progression-free survival in EGFR-overexpressing NSCLC patients treated with cetuximab plus chemotherapy.
ADC Development for NSCLC
ADC targeting NSCLC is to deliver potent agents such as auristatin, maytansinoid derivatives to the site expressing the target antigen, thereby reducing systemic toxicity while enhancing target cell killing activity. Antigen-specific ADC, in monotherapy or in combination with the current cancer therapies including immune checkpoint inhibitors, could provide new options for treating NSCLC with a high unmet medical need. Many ADCs have achieved a good anti-tumor activity in clinical trials and preclinical trials for the treatment of NSCLC, such as BAY 1129980 (anti-C4.4A), HcHAb18-DM1 (anti-CD147), U3-1402 (anti-HER3), SGN-15 (anti-Lewis Y antigen) and so forth. Creative Biolabs now provides customized ADC development services against the following antigens to help our clients boost their novel NSCLC therapy development.
Creative Biolabs has established unique platforms for every step in an ADC development. For instance, Antibody Design and Conjugation is used for generating new monoclonal antibodies with accommodating conjugation chemistry sites for the development of ADCs. DrugLnk is dedicated to the design and preparation for highly customized linker and drug-linker complexes. An effective combination of the most suitable antibody and linker-payload will greatly increase the chance for a successful ADC.
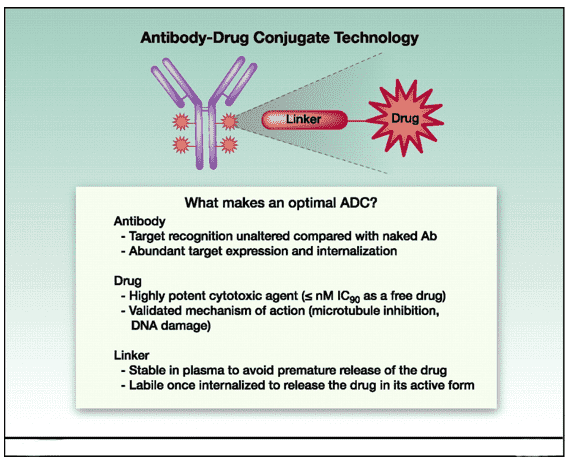 Fig.2 Schematic illustrating an ADC. (Teicher, 2011)
Fig.2 Schematic illustrating an ADC. (Teicher, 2011)
With a number of advanced technologies and scientific talents as well as rich experience in antibody production, antibody engineering, synthetic chemistry, and bio-conjugation, Creative Biolabs is dedicated to serving the specific needs of our clients in ADC development projects targeting NSCLC, and we are capable of providing high-quality services and products that are tailored to meet your R&D timeline and budget. Please contact us for more information and a detailed quote.
References
- Chan, B. A.; Hughes, B. G. M. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Translational lung cancer research. 2015, 4(1): 36.
- Teicher, B. A.; Chari, R. V. J. Antibody conjugate therapeutics: challenges and potential. Clinical cancer research. 2011, 17(20): 6389-6397.
For Research Use Only. NOT FOR CLINICAL USE.
Related Sections
ADC Applications in Diseases Research:
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

