Development of Biparatopic CD37-targeted Bispecific ADCs
Bispecific antibodies are artificial proteins comprised of two antibodies or antibodies fragments held together by a flexible peptide linker, which are presenting promising applications and outcomes in the field of cancer immunotherapy. Currently, several different bispecific antibodies are being researched and developed. Besides, antibody-drug conjugates (ADCs) have become more and more attractive as a therapeutic agent since eight ADC drugs have been approved for marketing presently. More importantly, recent research found that the combination of bispecific antibodies and ADCs is showing more potent anti-tumor activity owing to enhanced internalization and trafficking to the lysosome, and to maximize the amount of drug that is effectively delivered to tumor cells.
Creative Biolabs is a leading service provider in the field of ADCs development for cancer therapy. At present, we offer one-stop bispecific biparatopic ADCs design and construction services targeting CD37 biomarkers. Our innovative linker-payload and effective bioconjugation platforms will help your drug discovery and development.
Introduction of CD37
CD37 (leukocyte antigen CD37) is a heavily glycosylated protein, which is encoded by the CD37 gene in humans. It is a tetraspanin molecule that belongs to the family of the tetraspanins, a group of transmembrane proteins involved in cell membrane organization and co-signaling. CD37 is highly expressed on mature B-cells, but also, at lower levels, in T cells, macrophages/monocytes, granulocytes, and dendritic cells, and absent on normal stem cells and plasma cells.
CD37 can interact with many molecules, including PI3Kγ, PI3Kδ, SYK, LYN, CD19, CD22, SHP1, IL6R, SOCS3, CD53, CD81, CD82, major histocompatibility complex (MHC) class II, and integrins. CD37 may function in prosurvival and proapoptotic signaling. CD37 is highly expressed in mature B-cell lymphoid neoplasms such as chronic lymphocytic leukemia, which is also expressed on T-cell lymphomas. A wide range of CD37 positivity has been reported in diffuse large B-cell lymphoma (DLBCL). Therefore, CD37 has been considered as a target for B-cell lymphoma, especially for rituximab-resistant and relapsed patients. It was one of the first proteins explored as a therapeutic target in non-Hodgkin lymphomas in the late 1980s. Other new therapeutic agents targeting CD37 have been developed including naked monoclonal antibodies (BI836826), small modular immunopharmaceuticals (Otlertuzumab/TRU-016), ADCs (IMGN529 and AGS67E), and novel radioimmunoconjugates (177Lu Betalutin).
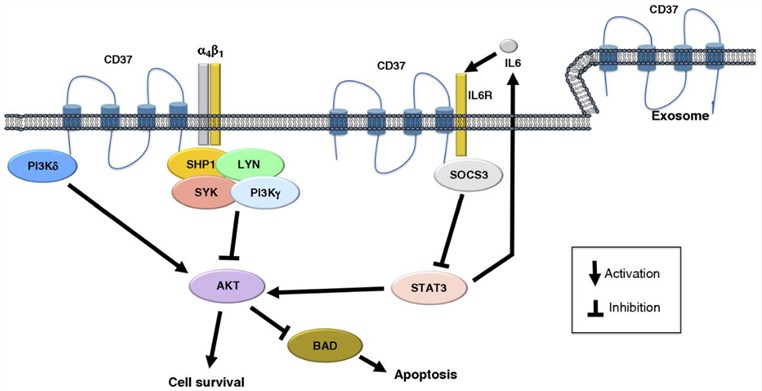 Fig.1 Schematic representation of the biologic role of CD37 in B cells. (Bertoni, 2016)
Fig.1 Schematic representation of the biologic role of CD37 in B cells. (Bertoni, 2016)
Biparatopic CD37-targeted Bispecific Antibody
DuoHexaBody-CD37 is a bispecific IgG1 antibody targeting two non-overlapping epitopes in the large extracellular loop of CD37, which possesses a hexamerization-enhancing mutation (E430G) in the IgG Fc domain to increases the intrinsic capacity of IgG1 molecules to form hexameric antibody clusters upon binding to membrane-bound antigens, thereby promoting the complement activation and CDC efficacy in CD37-positive B-cells. Using a broad range of B-cell lymphoma and leukemia patient samples (DLBCL, Follicular lymphoma, Mantle cell lymphoma, Marginal zone lymphoma, chronic lymphocytic leukemia) derived from both newly diagnosed and relapsed and/or refractory patients for the preclinical study, results revealed that DuoHexaBody-CD37 can induce potent CDC activity with median lysis of 82%, and significantly outperformed all three tested CD20 antibodies (rituximab, obinutuzumab, and ofatumumab). Thus, DuoHexaBody-CD37 has great therapeutic potential in B cell malignancies.
Bispecific biparatopic antibody against CD37 shows a strong capacity to induce CDC and kill cancer cells, which may be used in the ADC construction to achieve more effective anti-tumor activity combining the antibody induced CDC activity and small molecular drug cytotoxicity.
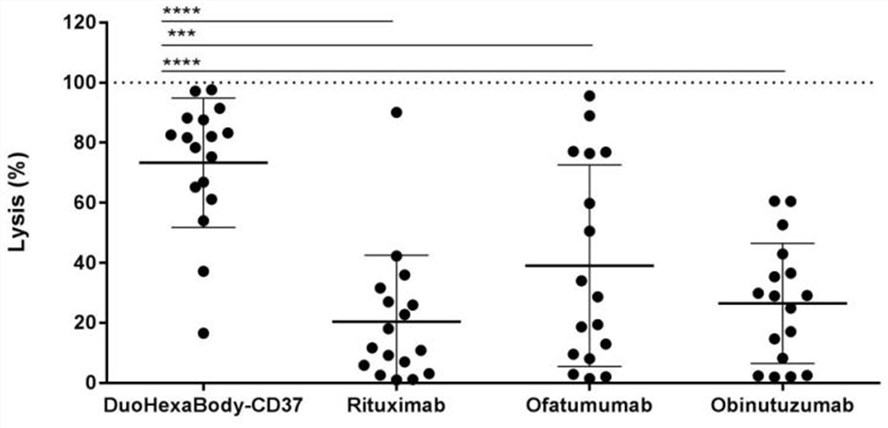 Fig.2 Complement-dependent cytotoxicity induced by DuoHexaBody-CD37, rituximab, ofatumumab, or obinutuzumab. (Van Der Horst, 2018)
Fig.2 Complement-dependent cytotoxicity induced by DuoHexaBody-CD37, rituximab, ofatumumab, or obinutuzumab. (Van Der Horst, 2018)
What Can We Do for You?
Empowered by advanced platforms and experienced technical personnel, Creative Biolabs offers comprehensive and coordinated services for the development, manufacture, and analysis of ADC. We now offer a whole suite of bispecific biparatopic ADCs development services targeting CD37 and our ADC development service include but not limited to:
- ADC Antibody Screening
- DrugLnk™ Custom Synthesis
- Antibody Design and Conjugation
- ADC in vitro Analysis
- ADC in vivo Analysis
If you are interested in our services, please contact us for more information.
References
- Bertoni, F.; Stathis, A. Staining the target: CD37 expression in lymphomas. Blood, The Journal of the American Society of Hematology. 2016, 128(26): 3022-3023.
- Van Der Horst, H. J.; et al. Targeting CD37 in B-Cell Malignancies Using the Novel Therapeutic Duohexabody-CD37 Results in Efficient Killing of Tumor B-Cells Ex Vivo Via Complement-Dependent Cytotoxicity, Even in Relapsed and/or Refractory Patient Samples. Blood. 2018, 132: 4179-4179.
For Research Use Only. NOT FOR CLINICAL USE.
Related Sections
Bispecific ADCs Development: Services:
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

