Immunomedics is a clinical biopharmaceutical company specializing in the development of monoclonal antibody-based therapies for the targeted treatment of cancer and other serious diseases. Recently, the company’s antibody-drug conjugate (ADC) sacituzumab govitecan (R&D code: IMMU-132), which has attracted the attention of the industry, has been hit hard in the United States in terms of regulation. The United States FDA sent a complete response letter (CRL) to the drug to treat metastatic triple-negative breast cancer (mTNBC) biological product licensing application (BLA). In CRL, the FDA refused to approve sacituzumab govitecan, on the grounds of creating problems, further delaying the drug’s regulatory timetable.
Introduction to Sacituzumab govitecan
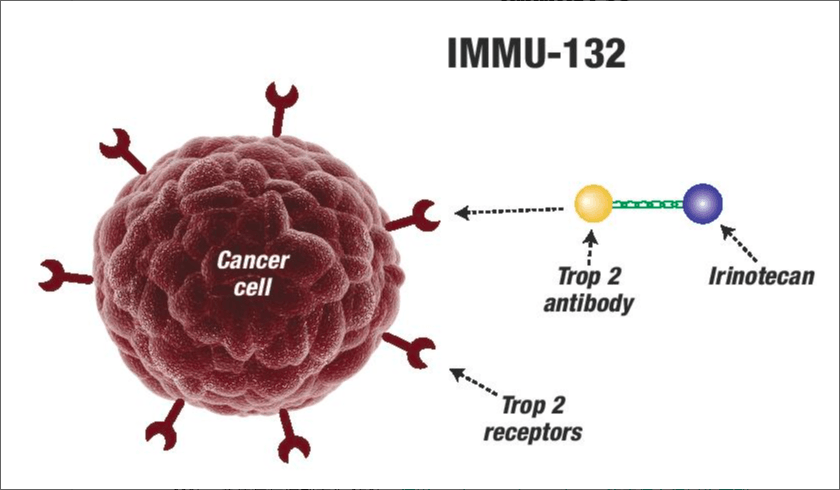
Sacituzumab govitecan is a new and first antibody-drug conjugate (ADC) drug, which is formed by coupling humanized IgG1 antibody targeting TROP-2 antigen with SN-38, a metabolite of irinotecan (a topoisomerase I inhibitor). TROP-2 is a cell surface glycoprotein expressed in more than 90% of TNBC.
The BLA, submitted by Immunomedics is intended to apply for FDA accelerated approval of sacituzumab govitecan for mTNBC patients who have previously received at least two treatments for metastatic conditions. Previously, the FDA had prioritized the drug BLA based on data from an ongoing single-arm II study.
Industry analysts had previously believed that, according to available II clinical data, sacituzumab govitecan was a significant improvement over standard care and, if approved, would be the first and only ADC drug to treat mTNBC. Sales are expected to peak at more than $1 billion.
In its last update in December, Immunomedics reported data from a phase II study of 108 patients in the group. As assessed by the Independent Review Committee, the total remission rate induced by sacituzumab govitecan was 34%, and the median remission duration was 9 months. At present, Immunomedics is also conducting a validated III phase clinical study of sacituzumab govitecan in the treatment of mTNBC.
Michael Pehl, President and Chief Executive Officer of Immunomedics, said, “We believe that sacituzumab govitecan has the potential to be a viable treatment for these patients. The issues related to approvals in this CRL focus only on chemistry, manufacturing, and control, and do not require the generation of new clinical or preclinical data. We will request a meeting with the FDA as soon as possible to fully understand FDA requirements and approval timetables, and we will work closely with FDA to bring this important drug to patients as soon as possible.
In addition to mTNBC, Immunomedics is planning clinical studies of sacituzumab govitecan in the treatment of other cancers. Two studies are recruiting patients: the critical II study (NCT03547973) is recruiting patients with metastatic urothelial carcinoma (mUC) who have failed to receive platinum-containing chemotherapy or PD- (L)-1 therapy. The Phase II study (NCT03725761) is recruiting patients with metastatic castrated resistant prostate cancer (mCRPC) who have progressed to the second generation of androgen receptor targeted therapy.
Introduction of TNBC
Breast cancer is the most common type of cancer among women, with more than 2 million confirmed cases worldwide each year. Triple-negative breast cancer (TNBC) accounts for about 15 percent of all breast cancer, and TNBC is more common in women under the age of 50 than other types of breast cancer. TNBC refers to breast cancer with negative expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2). The prognosis is very poor and the 5-year survival rate is less than 15%. TNBC is ineffective for hormone therapy and HER2 targeted therapy (such as Roche Herceptin), and the clinical treatment options are very limited, mainly relying on chemotherapy.
It is worth mentioning that the FDA is also giving priority to the BLA of Roche PD-L1 tumor immunotherapy Tecentriq combined with chemotherapy (Abraxane) in the first line treatment of PD-L1 positive locally advanced or metastatic TNBC. A final review decision is expected on 12 March 2019. If approved, the combination of Tecentriq and Abraxane will be the first cancer immunotherapy regimen to treat PD-L1-positive metastatic TNBC.