Recently, Rakuten Aspyrian, a clinical stage biotechnology company, announced a total of $284 million in Series C funding round with its antibody-drug conjugates based on photoimmunotherapy, including $134 million jointly funded by Rakuten and the new investor SBI Group in C+ round financing, plus $150 million won in August 2018. Aspyrian has raised approximately $352 million since its inception.
Aspyrian is currently sponsoring an ASP-1929 global phase 3 multicenter clinical trial called LUZERA-301 to evaluate the efficacy and safety of ASP-1929 in patients with recurrent head and neck cancer, as well as other phase 2 studies to treat other cancer types.
It is reported that Series C financing will further support the advancement of Aspyrian’s ASP-1929 project, currently in Phase 3 clinical trials for the treatment of patients with recurrent locally head and neck squamous cell carcinoma (HNSCC). Meanwhile, Aspyrian plans to launch Phase 2 study for other solid tumors in early 2019. If ASP-1929 is approved in the US, Japan and Europe, the financing will be used for the production and commercial development of the late ASP-1929.
PIT—Novel Accurate Cancer Treatment
The main technology of Aspyrian is Photoimmunotherapy (PIT), a new type of tumor-targeted anti-cancer platform, which combines the key advantages of antibody-mediated cancer cell targeting to achieve high tumor specificity, as well as laser-activated biophysical mechanisms that accurately induce rapid cancer cell death.
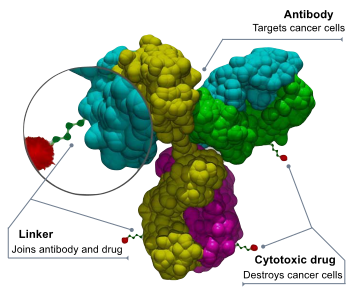
PIT consists of photoactivated antibody conjugates that target cancer and light application systems at the tumor site. The light application system includes a laser device and a light diffuser to irradiate the tumor using normal red light below a thermal threshold. Red light activates and triggers the pharmacological activity of the drug, leading to acute tumor necrosis.
PIT was originally developed by the National Cancer Institute and was exclusively licensed by Aspyrian. Currently, Aspyrian aims to commercialize multiple PIT-based therapies to combine a single drug that targets multiple solid tumors with other immunomodulators to produce a robust and long-lasting anti-cancer immune response.
Introduction to ASP-1929
ASP-1929 is the main product based on PIT of Aspyrian. It is an antibody-drug conjugate of cetuximab and IRDye700DX® that targets epidermal growth factor (EGFR) expressed in many types of solid tumors, including head and neck squamous cell carcinoma, esophageal cancer, and lung cancer, colorectal cancer, pancreatic cancer and other cancers.
ASP-1929 is locally activated with red light using proprietary research lasers and fibers after the antibody binds to the tumor. Local activation of tumor-selective conjugates targets tumors rather than normal tissues and structures. ASP-1929 was awarded a fast track by the FDA for the treatment of Head and Neck squamous cell carcinoma (HNSCC).
Compared to the historical data from the standard therapy, the midterm results of the 1/2 phase trial of HNSCC patients (RM-1929/101) showed an improvement in the objective response rate (ORR), progression-free survival (PFS) and overall survival (OS).