Roche recently announced that the Food and Drug Administration (FDA) has accepted the biological product licensing application (BLA) of the antibody drug conjugate (ADC) Polatuzumab Vedotin and granted priority review qualifications for it. The BLA approved Polatuzumab Vedotin combined with Bendamustine and Rituxan for the treatment of patients with recurrent or refractory diffuse large B-cell lymphoma (R/R DLBCL).
FDA has set a target date of August 19, 2019, for Prescription Drug User Fee Act (PDUFA). Previously, Polatuzumab Vedotin was granted orphan drug qualification by FDA and EU EMA, as well as breakthrough drug qualification (BTD) and priority drug qualification (PRIME), respectively.
The priority eligibility of FDA is granted to drugs that may provide significant improvements in the safety and effectiveness of the treatment, prevention or diagnosis of serious diseases. BTD is a new drug review channel created by FDA to accelerate the development and review of new drugs for the treatment of serious or life-threatening diseases, and there have preliminary clinical evidence that the drug can substantially improve the condition compared with existing therapeutic drugs. PRIME is a rapid approval project launched by EMA in March 2016, and it aims to accelerate the development and approval of truly innovative drugs to meet the medical demand for promising new drugs.
Introduction to Polatuzumab Vedotin
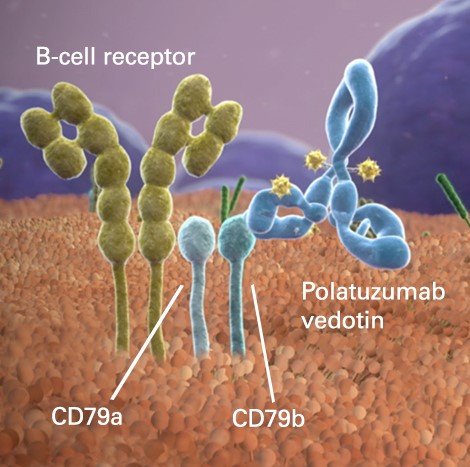
Polatuzumab vedotin, a kind of first-in-class anti-CD79b ADC, is formed by anti-CD79b antibody coupling with anti-mitogen MMAE. The highly specific expression of CD79b in most types of B-cell non-Hodgkin’s lymphoma (NHL) makes it an attractive target for the development of new therapies. Polatuzumab vedotin targets and destroys B cells expressed CD79b, maximizing the killing of cancer cells while minimizing side effects on normal cells while.
The BLA of Polatuzumab vedotin is based on positive data from a randomized phase Ib/II clinical study GO29365 (NCT02257567). The study was carried out in histologically confirmed R/R follicular lymphoma patients with (FL) or DLBCL, evaluating the efficacy and safety of intravenous infusion of polatuzumab vedotin combined with standard dose benzodipine (B) and rituximab (R) three-drug regimen (PBR) compared with standard dose benzodipine and rituximab second-drug regimen (BR).
A total of 80 patients with R/R DLBCL who had previously been over pretreated were enrolled in the II part of the study and were not eligible for hematopoietic stem cell transplantation. The data showed that compared with the BR two-drug regimen, the PBR three-drug regimen significantly prolonged the overall survival (median OS:12.4 months vs 4.7 months, HR=0.42, 95%CI:0.24-0.75; exploratory endpoint). In addition, compared with the BR two-drug regimen, the PBR three-drug regimen also significantly increased the complete remission rate (CR:40% vs 18%, primary endpoint), significantly reduced the risk of disease progression or death by 66% (median PFS:7.6 months vs 2.0months). HR=0.34, 95%CI: 0.20-0.57), and significantly prolonged the duration of mitigation (median DOR: 10.3 months vs 4.1 months, HR=0.44).
The medians of R/R DLBCL patients previously treated was 2. (PBR program group scope: 1-7; Br program group scope: 1-5). Subgroup analysis showed that compared with BR two-drug regimen, PBR three-drug regimen, whether as second-line, third-line and multi-line treatment, could benefit the survival of R/R DLBCL patients.
In terms of safety, the most common grade 3-4 adverse events was infection and hemocytopenia. Compared with BR, the incidence of grade 3-4 hemocytopenia was higher in PBR three-drug regimen. However, the infection rate and infusion rate between the two groups were similar.
DLBCL is the most common non-Hodgkin’s lymphoma (NHL), accounting for about 1/3 of NHL cases. DLBCL is an invasive (rapidly proliferating) type of NHL that recurs in up to 40 percent of patients and has a poor prognosis. It is estimated that more than 22000 new cases of DLBCL will be added in the United States in 2019. If approved, the PBR three-drug regimen will provide an important new treatment option for a significant prolongation of survival in the R/R DLBCL population.