Breast cancer is the most common cancer and cause of death among women. About 20% of breast cancers are HER2-positive (IHC3+ or IHC2+/ISH+), and the remaining 80% are HER2-negative. HER2 is a tyrosine kinase receptor growth-promoting protein expressed on the surface of certain cancer cells and is associated with progression and poor prognosis. At present, several HER2-targeted therapies have been approved for the treatment of HER2-positive metastatic breast cancer, which have improved the survival rate of patients.
However, approximately half of HER2-negative breast cancers still express a certain level of HER2 as a cell surface antigen and there is no anti-HER2 therapy and targeted treatment available for the HER2 low expression (IHC 2+/ISH- or IHC 1+) breast cancer.
Research Report
Daiichi Sankyo recently announced the latest data of safety and validity of an ongoing Phase I clinical study on the experimental HER2 targeting antibody drug conjugate (ADC) DS-8201 (trastuzumab deruxtecan).
In this study, a total of 54 HER2 low-expression breast cancer patients of overtreatment (The median number of previously accepted cancer protocols was 7.5) received at least 1 DS-8201 (dose 5.4 or 6.4 mg/kg) treatment. Until October 12, 2018, 23 patients are still receiving treatment.
The latest analysis of 43 patients with HER2-low-overexpressing metastatic breast cancer (IHC 2+/ISH- or IHC 1+) showed a overall response rate of the recommended extended dose of 5.4 or 6.4 mg/kg DS-8201 was 44.2% (n=19/43) and the disease control rate (DCR) was 79.1% (n=34/43). The initial estimated duration of response (DOR) was 9.4 months (range: 1.5+, 23.6+), and the median progression-free survival (PFS) was 7.6 months (95%). CI: 4.9, 13.7).
In this study, a total of 45 breast cancer patients with HER2 low-expression, hormone-receptive (HR+) received at least 1 DS-8201 (dose 5.4 or 6.4 mg/kg). Until October 12, 2018, 21 patients were still receiving treatment.
A further subgroup analysis of 38 evaluable patients with low HER2 expression and HR+ showed a total response rate of 47.4% (n=18/38) at the recommended extended dose of 5.4 or 6.4 mg/kg DS-8201, and the disease control rate was 81.6% (n=31/38). The median duration of response was 11.0 months (range: 1.5+, 23.6+) and median progression-free survival was 7.9 months (95% CI: 4.4, 13.7).
Shanu Modi, a breast cancer oncologist at the Sloan Kettering Cancer Center in the United States, said that, “Although anti-HER2 therapy plays an important role in the treatment of HER2-positive breast cancer, historically, this type of therapy has no effect on tumors with low HER2 expression. The published data provide primary evidence of the efficacy of DS-8201 in the treatment of patients with low HER2 breast cancer. Based on further research, we began to consider how to separate and treat these patients.”
According to Dr. Gilles Gallant, “There is currently no approval for anti-HER2 therapy for HER2 low-expression breast cancer, which represents about half of breast cancer cases. Based on this analysis, we have planned to launch a phase III clinical trial in which DS-8201 treats patients with HER2-low-metastasis breast cancer to expand the clinical development of DS-8201 for the treatment of HER2-expressing breast cancer and other tumor types.”
Introduction to DS-8201
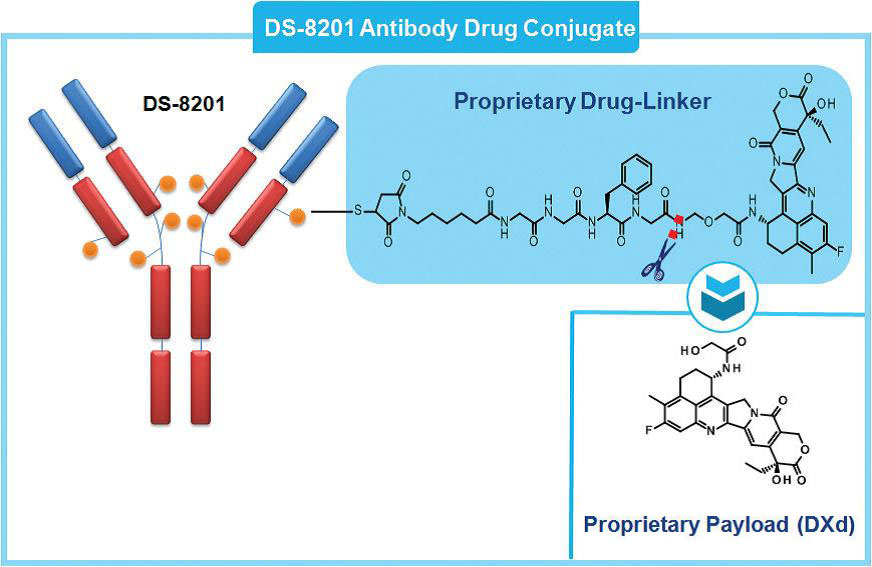
DS-8201 is an ADC drug that connects the humanized monoclonal antibody trastuzumab targeting HER2 and a novel topoisomerase 1 inhibitor, exatecan derivatives (DX-8951 derivatives, DXd) by a peptide linker to target the delivery of cytotoxic agents into cancer cells, which reduces systemic exposure of cytotoxic agents compared to conventional chemotherapy. Currently, DS-8201 is being developed for the treatment of various types of cancer, including breast cancer, stomach cancer, colorectal cancer, and bladder cancer.