In the ever-evolving landscape of oncology, the integration of targeted therapies and immunotherapy has emerged as a transformative strategy, particularly in breast cancer. Antibody-drug conjugates (ADCs), celebrated for their precision in delivering cytotoxic payloads to cancer cells, are now being explored alongside immune-checkpoint inhibitors (ICIs) to amplify antitumor responses. This partnership leverages ADCs’ dual role in direct cell killing and immune system activation, offering new hope for patients with challenging subtypes like triple-negative and HER2-positive breast cancer.
Understanding ADCs: A Blend of Targeting and Toxicity
ADCs represent a sophisticated fusion of monoclonal antibodies, chemical linkers, and cytotoxic payloads. The antibody component binds to specific antigens on cancer cell surfaces, such as HER2 in HER2-positive tumors or TROP-2 in triple-negative breast cancer (TNBC), enabling internalization of the ADC. Once inside the cell, the linker releases the payload, which disrupts vital cellular processes—such as DNA damage or microtubule function—leading to cancer cell death (Fig. 1).
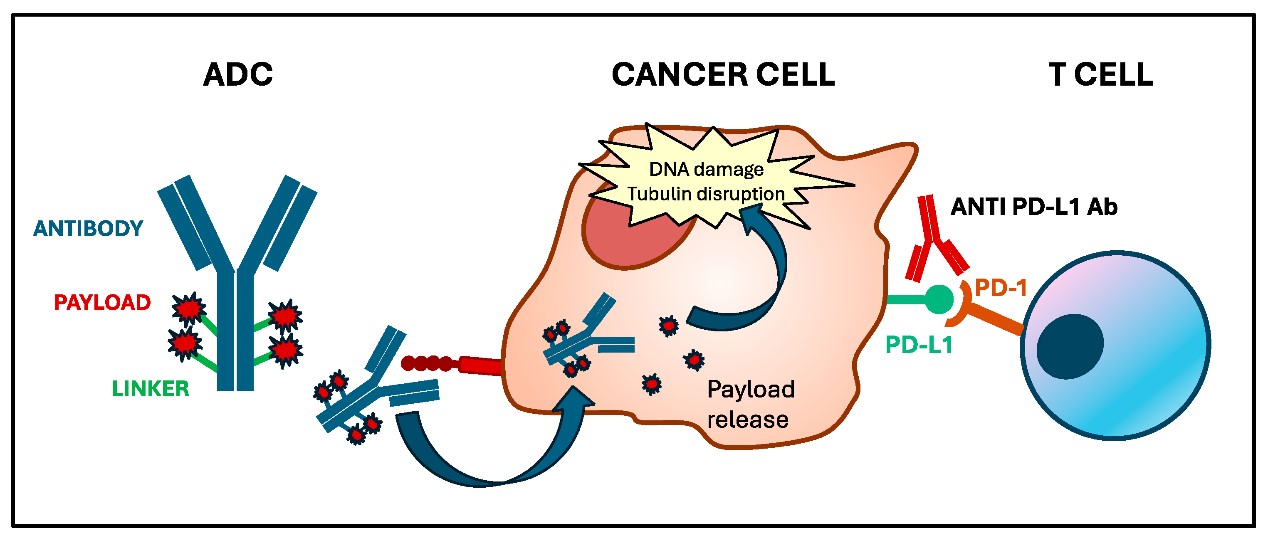 Fig. 1 Mechanism of action of antibody-drug conjugates. 1
Fig. 1 Mechanism of action of antibody-drug conjugates. 1
But ADCs are more than just precision-guided missiles. Preclinical studies reveal their ability to modulate the tumor microenvironment (TME), turning “cold” tumors into “hot” ones primed for immune attack. For example, ADCs can induce immunogenic cell death (ICD), a process where dying cancer cells release tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs). These molecules act as signals, recruiting dendritic cells to process and present TAAs to T cells, thereby activating both cytotoxic CD8+ T cells and helper CD4+ T cells. Additionally, ADCs can enhance the expression of PD-L1 on cancer cells, making them more susceptible to ICIs that block immune checkpoints like PD-1/PD-L1.
The Immune-Enhancing Potential of ADCs
ADCs reshape the TME in multiple ways, fostering an environment conducive to immune surveillance:
- Bystander Effect: Even cancer cells lacking the target antigen can be killed if they are near ADC-bound cells, thanks to payloads that diffuse within the TME.
- Macrophage Reprogramming: ADCs alter the activity of tumor-associated macrophages (TAMs), shifting them from a pro-tumor to an anti-tumor phenotype, which enhances phagocytosis and cytokine release.
- T Memory Cell Generation: Preclinical models show that ADCs can induce long-lasting immunity, with mice treated with HER2-targeting ADCs rejecting re-inoculated cancer cells, a sign of durable immune memory.
- Cytokine Recruitment: Dying cells release chemokines that attract natural killer (NK) cells and T cells, amplifying the immune response.
These mechanisms create a foundation for synergy with ICIs, which unleash the immune system by blocking inhibitory pathways. For instance, anti-PD-L1 antibodies prevent tumor cells from evading T cells, while ADCs ensure those T cells have a stronger signal to recognize and attack cancer.
Biomarkers: Guiding the Path to Personalized Combinations
Identifying which patients will benefit most from ADC-ICI combinations requires exploring predictive biomarkers, beyond just target antigen expression:
- Target Antigen Levels: While HER2 expression strongly correlates with response to HER2-targeting ADCs like trastuzumab conjugates, Trop-2-targeting ADCs (e.g., sacituzumab govitecan) show activity irrespective of Trop-2 levels, indicating alternative mechanisms at play.
- Tumor-Infiltrating Lymphocytes (TILs): High TILs are associated with better responses to ICIs, but their role in ADC efficacy is nuanced. In neoadjuvant settings, elevated TILs predicted pathologic complete response (pCR) in TNBC patients treated with sacituzumab govitecan, suggesting TILs may mark tumors primed for immune activation by ADCs.
- Neutrophil-to-Lymphocyte Ratio (NLR): A low baseline NLR, a systemic marker of inflammation, correlates with improved progression-free survival (PFS) and overall survival (OS) in HER2-positive patients on ADCs, highlighting the importance of host immune status.
- PD-L1 Expression: While PD-L1 positivity has guided ICIs in TNBC, its role in ADC combinations is mixed. Subgroup analysis from the KATE 2 trial showed a trend toward improved PFS in PD-L1-positive HER2+ metastatic breast cancer patients treated with T-DM1 plus atezolizumab, though larger studies are needed to confirm this.
Clinical Trials: Promising Results and Ongoing
Early-phase trials have demonstrated the potential of ADC-ICI combinations, with encouraging response rates and manageable toxicity:
- HER2-Positive Breast Cancer: The phase Ib study of T-DM1 plus pembrolizumab in metastatic HER2+ breast cancer reported an overall response rate (ORR) of 20%, with median PFS of 9.6 months. Meanwhile, the KATE 2 trial, though negative in the overall population, showed a significant trend toward benefit in PD-L1-positive subgroups, leading to the ongoing phase III KATE 3 trial focusing on this subset.
- Triple-Negative Breast Cancer (TNBC): The BEGONIA trial made waves with its arm testing datopotamab deruxtecan (Dato-DXd) plus durvalumab, achieving an impressive ORR of 79% in metastatic TNBC, regardless of PD-L1 expression. Sacituzumab govitecan combined with pembrolizumab is being tested in the phase II SACI-IO trial, aiming to improve outcomes in PD-L1-negative TNBC, a group that has historically derived less benefit from ICIs alone.
- Neoadjuvant Setting: In early-stage HER2+ breast cancer, the ASTEFANIA trial is evaluating whether adding atezolizumab to adjuvant T-DM1 can improve invasive disease-free survival (IDFS) in patients with residual disease after neoadjuvant therapy. Similarly, the NeoSTAR trial showed that neoadjuvant sacituzumab govitecan induced pCR in TNBC, with TILs emerging as a key biomarker of response.
These trials highlight the importance of patient selection: while HER2-targeting ADCs thrive in HER2+ tumors, Trop-2-targeting ADCs offer promise across HER2-low and TNBC, expanding the therapeutic landscape.
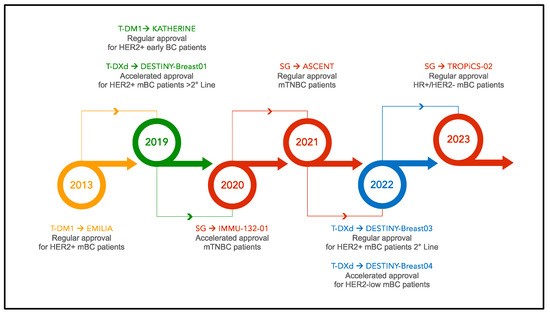
Fig. 2 Timeline of ADC approval in breast cancer. 1
Creative Biolabs is a leading biotech company specializing in antibody-drug conjugate (ADC) development services, offering comprehensive one-stop solutions for breast cancer-related ADC research. With advanced technology platforms and a team of experienced scientists, the company provides customized services across the entire ADC development pipeline, from antibody screening and design to in vitro/in vivo analysis and manufacturing.
| ADC Targeting HER2 | ADC Targeting CA6 |
| ADC Targeting Notch3 | ADC Targeting P-Cadherin |
| ADC Targeting LIV1 | ADC Targeting GPNMB |
Challenges and Future Directions
While ADC-ICI combinations show promise, key challenges remain. Toxicity management is critical: ADCs pose risks like hematological or neurological issues, while ICIs carry immune-related side effects, though early data suggest manageable overlaps. Biomarkers like TILs and NLR need validation in large studies, with multi-omics approaches poised to identify more precise predictors. Optimal treatment sequencing (e.g., neoadjuvant vs. metastatic use) and dosing schedules are being explored in trials like I-SPY 2. Overcoming resistance—from reduced antigen expression or MHC loss—requires deeper mechanistic research, though combinations may delay pathway-specific evasion.
The Road Ahead
This therapeutic pairing represents a paradigm shift, leveraging ADCs’ dual cytotoxic and immune-modulating effects with ICIs’ immune activation. Ongoing phase III trials (e.g., KATE 3, ASCENT-05) signal a future of personalized, synergistic care, particularly for HER2+ and TNBC subtypes. For patients, this means hope for prolonged remissions; for researchers, unraveling tumor-immune interactions will drive precision oncology closer to reality.
References
- Nucera, Sabrina, et al. “Antibody-drug conjugates to promote immune surveillance: Lessons learned from breast cancer.” Biomedicines7 (2024): 1491. https://doi.org/10.3390/biomedicines12071491