ADC Development Services Targeting HER2
Combining the ability of monoclonal antibodies (mAbs) to specifically target tumor cells with the highly potent killing activity of payload drugs, antibody-drug conjugates (ADCs) have emerged as a powerful strategy in cancer therapy. Targeting the human epidermal growth factor receptor 2 (HER2) has yielded major advances in HER2 positive cancer treatments. Creative Biolabs now offers a variety of quallfied anti-HER2 ADC Products and customized ADC development services to serve your course of ADC development project.
Introduction of HER2
HER2 is a member of the human epidermal growth factor receptor (EGFR) family, which also comprises EGFR, HER3 and HER4. HER2 is aberrantly expressed in certain malignancies, including breast and gastric cancer, primarily due to HER2 genomic amplification. HER2 expression can be detected on cell membranes of epithelial cells in the gastro-intestinal tract, reproductive tract, respiratory tract, urinary tract, skin, breast and placenta. A HER2 amplification can promote tumorigenesis through multiple mechanisms and can therefore be considered as an oncogenic driver in HER2 amplified cancers. Besides breast cancer, HER2 was also found to be amplified and/or overexpressed in several cancer types including gastric and lung cancer. Dysregulated HER2 expression is associated with increased risk of recurrence and poorer prognosis in these cancers. In HER2-positive malignancies, HER2 can stimulate downstream RAS-RAF-ERK and PI3K-PTEN-AKT signaling and play a key role in cell proliferation. Therefore, HER2 is the preferred therapeutic target in such cancers.
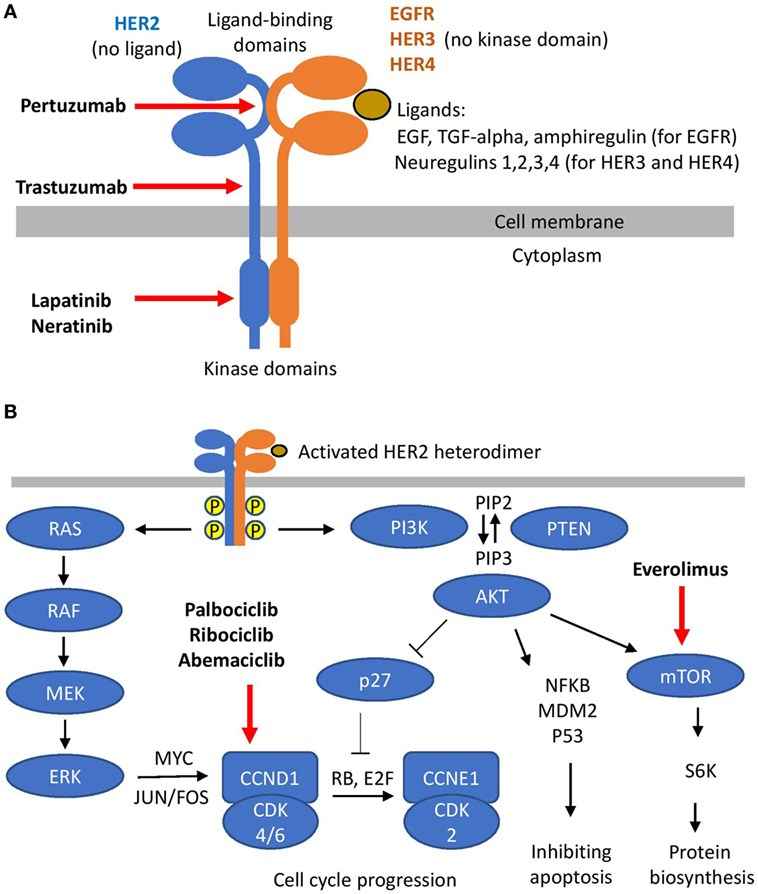 Fig.1 HER2 and its targeting.1,3
Fig.1 HER2 and its targeting.1,3
Anti-HER2 ADC in Breast Cancer
Traditionally, HER2-positive breast cancer was regarded as the most aggressive subtype. A high rate of recurrences were observed before the introduction of anti-HER2 targeted therapies. The HER2-targeted agents currently approved for treatment of metastatic breast cancer include trastuzumab, pertuzumab, lapatinib, and trastuzumab-emtansine (T-DM1). T-DM1 is a HER2-targeting ADC comprising the antibody trastuzumab and the tubulin inhibitor DM1 conjugated via a linker. Although T-DM1 is effective in treating advanced breast cancer, all patients eventually develop T-DM1 resistance. Trastuzumab deruxtecan (DS-8201) is a new ADC comprised of a humanized anti-HER2 antibody, a newly developed, enzymatically cleavable peptide linker, and a novel, potent, exatecan-derivative topoisomerase I inhibitor. DS-8201 has a drug-to-antibody-ratio (DAR) of 8, which is higher than that of T-DM1. Both T-DM1 and DS-8201 had a manageable safety profile and showed preliminary activity in heavily pretreated patients with HER2-positive breast cancer, gastric or gastro-oesophageal junction cancer. Other ADCs under developing include:
- XMT-1522 is a new ADC and its antibody part targets a new epitope on HER2, thus avoiding potential competition for epitopes with trastuzumab or pertuzumab if used in combination. XMT-1522 showed significantly higher potency than T-DM1 in preclinical models. Currently, it is being tested in a phase I clinical trial (NCT02952729).
- The ADC A166 is composed of a monoclonal anti-HER2 antibody conjugated to a cytotoxic agent. A166 is currently investigated in a running phase 1/2 trial including patients with relapsed or refractory HER2 expressing or HER2 amplified cancers.
- ALT-P7 is an ADC composed of the trastuzumab biobetter HM2 conjugated in a site-specific manner to monomethyl auristatin E (MMAE). ALT-P7 is currently investigated in an ongoing open-label, dose escalation and phase 1 trial in patients with HER2 positive metastatic breast cancer.
- ADC ARX-788 is composed of a monoclonal HER2 targeting antibody site-specifically conjugated, via a non-natural amino acid linker para-acetyl-phenylalanine (pAcF), to monomethyl auristatin F (MMAF). The site-specific conjugation of MMAF to the HER2 antibody improves the therapeutic window of ARX-788 by increasing payload stability and optimizing its half-life. ARX-788 is currently investigated in two ongoing Phase 1 trials. Besides the mentioned ADCs, there are also several ADCs currently investigated in ongoing clinical trials such as DHES0815A, RC48, SYD985, MEDI4276 and XMT-1522.
Anti-HER2 ADC in Gastrointestinal Malignancies
Gastrointestinal cancers represent one of the most common cancer types. HER2 is found to be present in nearly all histologic types of gastrointestinal cancers in variable degrees of expression. HER2 testing is necessary in daily practice for treatment of advanced gastrointestinal cancers patients. Progress have been made by scientists for HER2 targeted therapies in gastrointestinal cancers, including esophagogastric, pancreaticobiliary, and colon cancers. Similarities do exist between HER2-positive breast and gastric cancer, in terms of the incidence of HER2-positivity, pathologic concordance rates and until recently their management in the first-line metastatic setting. To date, DS-8201, ARX-788, MEDI4276, SYD985, XMT-1522 were also investigated in patients with advanced pretreated HER2 expressing gastric cancer.
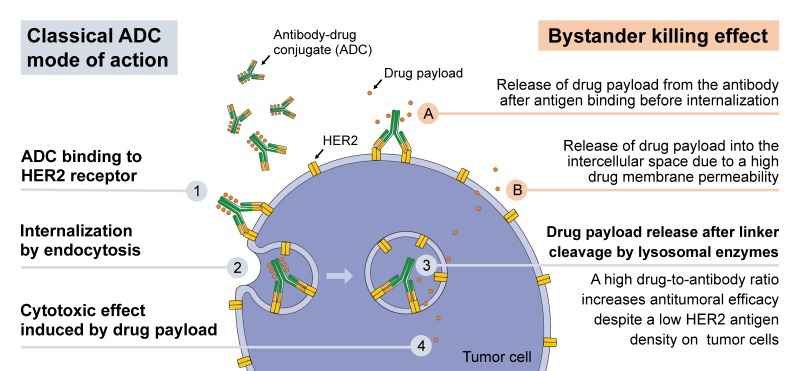 Fig.2 Mode of action of HER2 directed ADCs.2,3
Fig.2 Mode of action of HER2 directed ADCs.2,3
What Can We Do for You?
Having been engaged in ADC design and development for a long time, Creative Biolabs has recognized the promising applications of anti-HRE2 ADCs for breast cancers and gastrointestinal cancers research. It is known that the main directions in the development of new ADCs include new targeting antibodies, advanced linker technologies, as well as potent drug. Equipped with our ADC Antibody Screening technique, Creative Biolabs follows the three key elements of ADC development and provides Anti-HER2 Antibody Development services supplying mAbs targeting different epitopes on HER2.
Creative Biolabs can also improve the potency of anti-HER2 ADCs for our clients by substantially reducing their affinity for HER2 at acidic endosomal pH relative to near neutral pH. These engineered antibody variants show increased lysosomal delivery and cytotoxicity towards tumor cells expressing intermediate HER2 levels.
Additionally, Creative Biolabs has explored and adopted a variety of methodologies in Antibody Design and Conjugation to introduce a series of specific and chemically versatile conjugation sites into the antibody sequences to achieve a narrower DAR. Our Featured DrugLnk™ Custom Synthesis platform provides customized drug-linker complex synthesis services for ADC development. ADC in vitro Analysis and ADC in vivo Analysis services are also optional according to your demands.
If you are interested in our ADC development services targeting HER2, please feel free to contact us for more information.
References
- Larionov, Alexey A. "Current therapies for human epidermal growth factor receptor 2-positive metastatic breast cancer patients." Frontiers in oncology 8 (2018): 89..
- Rinnerthaler, Gabriel, Simon Peter Gampenrieder, and Richard Greil. "HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer." International journal of molecular sciences 20.5 (2019): 1115.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
