The FORWARD I Phase III registration trial (NCT02631876) evaluating the antibody-drug conjugate or ADC mirvetuximab soravtansine as a single-agent therapy for the treatment of platinum-resistant ovarian cancer, has completed full enrollment and will continue as planned without modification.
Mirvetuximab soravtansine, also known as IMGN853, is the first FRα-targeting ADC. The drug, which is being developed by clinical-stage biotechnology company Immunogen, uses a humanized FRα-binding antibody to target the ADC specifically to FRα-expressing cancer cells and a potent anti-tumor agent, DM4, to kill these targeted cancer cells.
Mirvetuximab is also being assessed in combination regimens for both platinum-resistant and platinum-sensitive disease in the Phase Ib/II FORWARD II trial.
The decision follows a recommendation by the Independent Data Monitoring Committee (IDMC) based upon successful completion of a pre-specified interim futility analysis after 80 progression-free survival (PFS) events as determined by blinded, independent central review.
Completed full enrollment of the trial was realized two months ahead of schedule. Researchers are expecting top-line results from FORWARD I sometime during the first half of 2019.
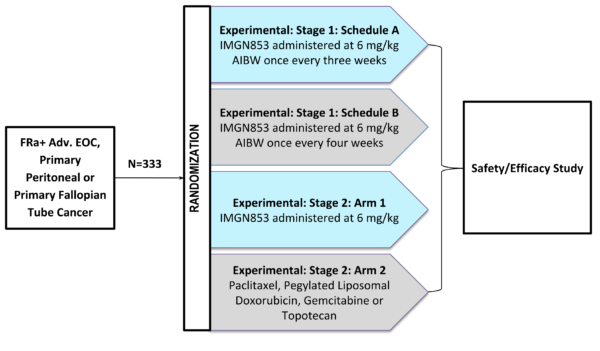
The Phase III, open label, randomized FORWARD I study (NCT02631876) was designed to compare the safety and efficacy of mirvetuximab soravtansine, also known as IMGN853, to that of selected single-agent chemotherapy (Investigator’s choice) in women with platinum-resistant FR-alpha positive advanced EOC, primary peritoneal cancer and/or fallopian tube cancer.
“We are encouraged that the IDMC recommended FORWARD I proceed as planned and are pleased that the trial has reached full enrollment earlier than expected. We look forward to assessing top-line data in the first half of 2019,” noted Anna Berkenblit, MD., vice president and chief medical officer of ImmunoGen.
Ovarian Cancer
“Ovarian cancer is the leading cause of death from gynecological cancers, and patients diagnosed with this life-threatening disease have limited treatment options, especially once they develop platinum-resistant disease,” Berkenblit explained.
It is estimated that 22,000 women are diagnosed annually with ovarian cancer in the US. With more than 14,000 deaths each year, ovarian cancer accounts for more deaths than any other cancer of the female reproductive system.
Standard first-line therapy for ovarian cancer is a platinum-based combination regimen. Once the cancer becomes platinum-resistant, treatment options include single-agent cytotoxic therapies such as pegylated liposomal doxorubicin, paclitaxel, or topotecan, and combination therapies that include bevacizumab.
There is a significant need for more effective, better-tolerated therapies for recurrent ovarian cancer. It is estimated that approximately 19,000 women in the US and approximately 24,000 women in the EU have platinum-resistant ovarian cancer requiring second-line or later treatment.
ImmunoGen estimates that 60% of ovarian cancer cases have medium or high FRα expression.
Trial design
FORWARD I is an ongoing Phase III trial designed to randomize 333 patients 2:1 to receive either mirvetuximab soravtansine or the physician’s choice of single-agent chemotherapy (pegylated liposomal doxorubicin, topotecan, or weekly paclitaxel). Eligibility criteria include patients with platinum-resistant ovarian cancer that express medium or high levels of folate receptor alpha (FRα) who have been treated with up to three prior regimens. The primary endpoint of this study is PFS, which is being assessed in the entire study population and in the subset of patients with high FRα expression. Enrollment was initially planned to be completed by the end of June.
ImmunoGen is partnering with the Gynecologic Oncology Group Foundation Inc., a leader in clinical research in gynecologic malignancies, on FORWARD I, which is being conducted in North America and Europe. This trial is intended to support full marketing approval of mirvetuximab soravtansine for patients with platinum-resistant ovarian cancer.
Source: https://adcreview.com/news/forward-i-phase-iii-trial-of-mirvetuximab-soravtansine-in-platinum-resistant-ovarian-cancer-completes-full-enrollment/