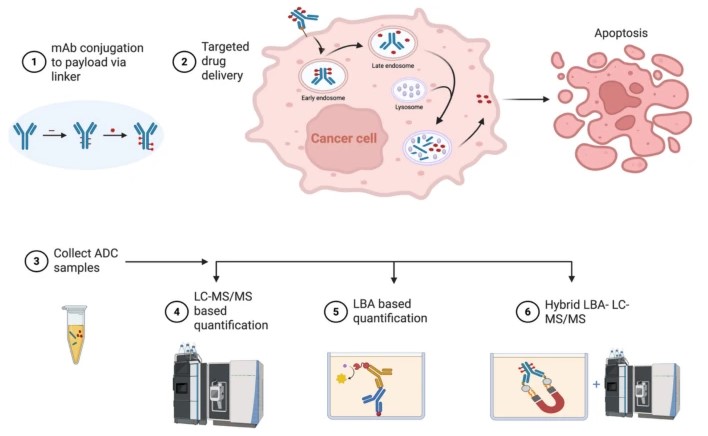
In the rapidly evolving landscape of oncology, Antibody-Drug Conjugates (ADCs) have emerged as one of the most promising classes of therapeutics. Often described as “biological missiles,” these sophisticated molecules represent a precise convergence of biology and chemistry, uniquely combining the target specificity of monoclonal antibodies with the potent killing power of cytotoxic small-molecule payloads.
With over 100 ADCs currently in clinical trials for various cancer treatments, the field is expanding at an unprecedented rate. However, the successful development of these complex therapeutics requires overcoming significant bioanalytical and pharmacological challenges. This article explores the latest scientific advancements in characterization and modeling that are driving the next generation of safer, more effective ADCs.
The Anatomy of a Therapeutic Hybrid
To appreciate the complexity of developing ADCs, one must first understand their architecture. An ADC is comprised of three critical components:
- The Antibody: Designed to recognize and bind to specific antigens on the surface of cancer cells.
- The Payload: A highly potent cytotoxic agent intended to induce cell death.
- The Linker: A chemical bridge that covalently attaches the payload to specific amino acids on the antibody.
The mechanism of action is a highly coordinated sequence. Upon binding to the target cell surface antigen, the ADC is internalized via endocytosis. It progresses from an early endosome to a late endosome, which eventually fuses with a lysosome. Within this acidic compartment, enzymes degrade the linker or the antibody, releasing the payload to trigger apoptosis.
A critical phenomenon in this process is the “bystander effect”. Some payloads are designed to be membrane-permeable. Once released inside the target cell, these molecules can diffuse outward to kill neighboring cells, including those that may not express the target antigen. While this can enhance efficacy in heterogeneous tumors, it also presents a risk of toxicity to surrounding healthy tissues, making the stability of the linker a paramount design consideration.
Fig.1 Schematic of antibody-drug conjugate cellular mechanisms and quantification bioanalytical strategies.1
The Bioanalytical Challenge: Taming Heterogeneity
A major hurdle in ADC development is inherent heterogeneity. During synthesis, payloads are conjugated to the antibody at various sites, creating a mixture of species with different Drug-to-Antibody Ratios (DAR), typically ranging from 0 to 8 drugs per antibody. Furthermore, once in circulation, the payload can “deconjugate” or detach, altering the drug’s safety profile.
To ensure safety and efficacy, researchers rely on three primary analytical pillars to characterize these dynamic mixtures:
- Advanced Ligand Binding Assays (LBAs)
Ligand binding assays remain a fundamental tool for quantifying total antibody and conjugated antibody levels. While traditional enzyme-linked immunosorbent assays (ELISA) are cost-effective and sensitive, they can suffer from matrix interference and limited dynamic range.
Recent innovations include electrochemiluminescence immunoassays (ECLIA), which offer wider dynamic ranges and higher sensitivity compared to traditional methods. Additionally, automated microfluidic immunoassay platforms have revolutionized the field by utilizing nanoliter-scale samples. These systems use centrifugal forces to control liquid flow through affinity columns, significantly reducing sample volume requirements and hands-on time while maintaining high precision.
- High-Resolution Mass Spectrometry (LC-MS/MS)
To achieve deeper structural insights, scientists utilize Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). This technology enables quantification at multiple levels:
- Top-down Analysis: Examines the intact ADC to determine the distribution of DAR species without digestion.
- Middle-down Analysis: Involves partial digestion into larger subunits (such as light and heavy chains) to localize conjugation sites.
- Bottom-up Analysis: Digests the protein into small peptides for precise, site-specific characterization.
Advancements in ionization techniques, such as nano-electrospray ionization, and the use of high-resolution mass analyzers (such as Fourier transform ion cyclotron resonance mass spectrometers), have dramatically improved sensitivity. These methods can now detect minute quantities of unconjugated payload in plasma, a critical safety check to ensure the toxic drug is not leaking prematurely.
- Hybrid Immunoaffinity LC-MS/MS
The emerging “gold standard” is the hybrid approach. This technique combines the selectivity of immunocapture (enriching the ADC from biological matrices) with the structural resolution of mass spectrometry. This method is particularly powerful for tracking biotransformations in vivo, revealing degradation products that traditional assays might miss.
Mapping the Journey: Pharmacokinetic (PK) Modeling
Because ADCs possess characteristics of both large and small molecules, their pharmacokinetic profiles are complex. The antibody component typically drives the half-life and distribution, while the payload influences toxicity. To navigate this, researchers employ sophisticated mathematical models:
- Population PK Models: These analyze data to understand how patient demographics (such as body weight or organ function) affect drug exposure, helping to optimize dosing regimens.
- Physiologically Based Pharmacokinetic (PBPK) Models: These mechanistic models simulate drug behavior in specific organs and tissues. They are increasingly used to predict potential drug-drug interactions without the need for dedicated clinical trials.
- Quantitative Systems Pharmacology (QSP): This advanced discipline integrates PK data with disease biology. QSP models can simulate cellular mechanisms—such as internalization rates and the bystander effect—to predict efficacy and safety outcomes in patients.
The Future: Dual-Payloads and Homogeneity
The next frontier in ADC development involves multi-payload conjugates. researchers are exploring strategies to attach two different cytotoxic agents, or a combination of a cytotoxic agent and an immunomodulator, onto a single antibody. This approach aims to overcome drug resistance by attacking tumors via multiple mechanisms simultaneously.
Concurrently, there is a significant push toward homogeneity. By refining conjugation chemistry, developers aim to create ADCs with a consistent, uniform DAR (e.g., exactly 8 drugs per antibody) rather than a heterogeneous mixture. These next-generation molecules theoretically offer more predictable pharmacokinetics and improved stability in the bloodstream.
Conclusion
The development of Antibody-Drug Conjugates represents a triumph of modern bioengineering. However, their structural complexity demands equally sophisticated analytical and mathematical tools. The integration of advanced bioanalytical platforms with mechanistic modeling is essential for understanding the behavior of these potent therapeutics. By refining these methods, scientists are streamlining the development process, ultimately paving the way for safer and more effective treatment options.
Creative Biolabs offers a comprehensive suite of ADC evaluation services to accelerate therapeutic development. Our platform spans in vitro analysis, including biochemical characterization and advanced 3D cell culture-based efficacy testing. We provide rigorous in vivo analysis focusing on pharmacokinetics (PK) characterization, safety assessments, and immunogenicity analysis to ensure clinical viability. By combining precision biochemical analysis with robust in vivo efficacy evaluation, we deliver the critical data needed to optimize ADC stability, potency, and safety profiles. Trust our expertise to transform your complex conjugates into successful clinical candidates.
Reference
- Khan, R. M., et al. “Recent advances in bioanalytical methods for quantification and pharmacokinetic analyses of antibody–drug conjugates.” The AAPS journal6 (2025): 1-15. CC BY 4.0. https://doi.org/10.1208/s12248-025-01115-9