On May 2, Johnson & Johnson, a pharmaceutical giant, announced that it has invested $1 billion in the acquisition of BeneVir Biopharm, a biotech-company dedicated to oncolytic virus immunotherapy research. This news makes oncolytic virus technology once again the center of public attention. Not long ago, on April 25, a review article titled Oncolytic viruses as engineering platforms for combination immunotherapy was published on Nature Review-Cancer. Combining this article, we can understand it more clearly that the important status of the oncolytic virus and the unlimited potential it can play in the future.
Immune checkpoint inhibitors, T cell therapy, and tumor vaccines have achieved tremendous success in clinical practice. At present, it is particularly important to design a rational combination therapy strategy, which can improve the efficacy and benefit more patients. For example, in the treatment of metastatic melanoma, the combined use of two drugs targeting PD1 and CTLA4 resulted in a two-fold increase in patient response. On the other hand, due to the heterogeneity of tumors, it is necessary to design a very large number of combinations to target different tumors, which also doubles the drug toxicity and treatment costs.
Oncolytic viruses (OVs) provide a perfect solution to this problem. It can be designed to replicate and kill tumor cells only in tumor tissue while activating the host’s own anti-tumor immune system. In this review, the authors comprehensively analyzed and discussed the prospects of oncolytic viruses as an “immune modification platform”: to allow the virus to express its own immune checkpoint inhibitors, tumor antigens, cytokines, and T cell adaptors, helping us to overcome the barrier of T cell recognize and attack tumor cell. This will serve as a new synergistic mechanism in the anti-tumor process.
About oncolytic virus
Oncolytic viruses are viruses that have been engineered or screened to selectively infect, expand and kill cancer cells. OVs can infect in situ and migrate in the human system, so they can function both in situ and in metastatic tumor sites. At present, a variety of OV targeting strategies have been intensively studied. A common principle of design is to delete the virulence factor gene of the virus. By using aberrant signal pathways in cancer cells, OVs cannot be replicated in normal cells, but keep the vitality of copy and killing in cancer cells. For example, the key viral defense mechanism in mammalian cells is mediated by interferons and cytokines. But this pathway fails in cancer cells, which provides conditions for virus replication only in cancer cells but not in normal cells. The modified rhabdoviruses VSV and Maraba are typical representatives of which rely on this pathway. The first oncolytic virus drug, Imlygic, approved by the FDA, derived from HSV-1 virus, was modified to remove both ICP34.5 and ICP47 genes. The former gene inhibiting the translation of cellular proteins, the latter inhibiting antigen presentation. Another oncolytic virus, Pexa-Vec, currently in clinical phase 3, has deleted the thymic acid kinase gene, allowing it to replicate only in cells with high kinase activity, such as liver cancer cells.
OVs can be treated with a single dose or combined with other immunotherapy to make the body’s immune system biased against tumors rather than against the virus itself. Because MHC molecule has a higher affinity for viral antigens than tumor antigens, and in OVs therapy, rhabdovirus-induced immunity is more likely to against virus than tumor cells. However, latest studies have found that pre-treatment with replication-defective oncolytic viruses expressing TAAs (tumor antigen-associated proteins) can be a good solution to this problem and make the immune system tend to be anti-tumor.
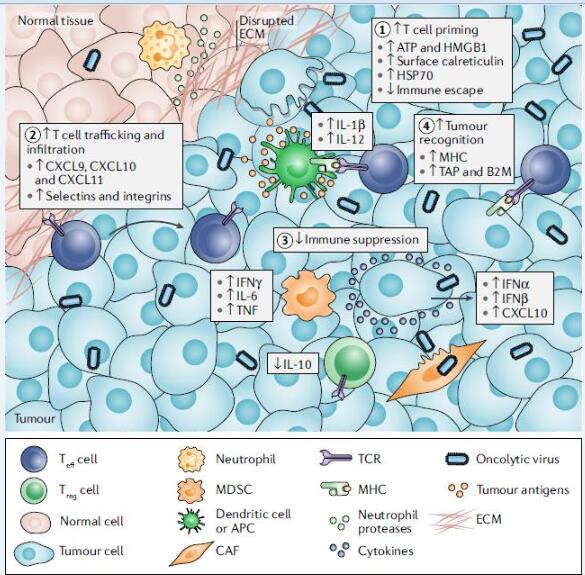
Figure 1. Mechanisms by which oncolytic viruses stimulate antitumour immunity. Oncolytic virus exerts its immune activity through four steps: 1. T-cell loading; 2. T-cell transport and infiltration; 3. Avoidance of immunosuppression; 4. Engagement of tumor cells;
- T cell loading
In any T cell response, the presentation of tumor-specific antigens to T cells containing homologous TCRs is prerequisite, and this process requires the participation of APCs (antigen presenting cells). The APC function in cancer tissue is often destroyed and TAAs cannot be presented. For example, the tumor’s intrinsic β-catenin oncogenic signaling pathway inhibits the recruitment of APCs at the tumor site. Using OVs as an in situ vaccine, OVs kill tumor cells and release intracellular TAAs, PAMPs, and DAMPs. Moreover, OV can infect APCs, promote their function maturation, and lead to type 1 interferon response. These inflammatory and antigenic stimuli lead to tumor-specific immune responses.
- T-cell transport and infiltration
Once recruited, T cells must be transported and infiltrated into the tumor site. The chemokines associated with T cell infiltration in human tumors are CXCL9, CXCL10, and CXCL11. Studies have shown that the expression levels of these factors are strongly correlated with the number of intratumoral T cells and the patient’s survival. OVs can promote T cell infiltration in tumors in many ways: First, it can cause a type 1 interferon response that stimulates the recruitment of T cells to recruit chemokines; Second, oncolytic viruses can induce inflammatory stimuli such as TNF and IL-1β, which in turn upregulate the expression of selectins in endothelial cells and promote T cell infiltration; Third, OVs can be designed to grow in tumor cells that have an immunosuppressive signaling pathway, turning the signaling pathway into a pro-inflammatory mediator; Fourth, OVs can be designed to encode T cell chemokines, providing a direct solution to the defects in genes and related genes at the apparent level in tumor cells.
- Avoidance of immunosuppression
Once immune cells reach the TME (Tumor Microenvironment), they must overcome the complex network of cellular and extracellular matrix (ECM) components. OVs can attract neutrophils, which can cause changes in the tumor microenvironment through inflammatory factors and host kinases. To enhance this effect, OVs can be designed to express ECM editors.
T cells that infiltrate tumors still need to fight immunosuppressive cells and other inhibitors in the tumor microenvironment (TME). Using OVS allows inert tumors to be sensitive to immune checkpoints again. Humans have evolved a network of immune checkpoints to limit the immune-induced pathological response, which may be particularly relevant to viral infections. Therefore, in OV-mediated oncolysis, interferon products and recruited T cells eventually target immune checkpoint inhibitors, limiting the inflammatory response. Therefore, the introduction of immune checkpoint inhibitors (ICIs, such as PD1, CTLA4) during the use of OVs can significantly improve the effect and prolong the patient’s survival.
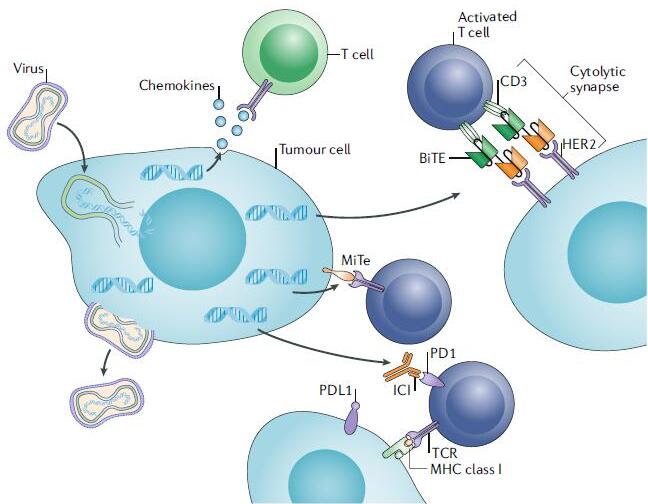
Figure 2. Design elements of a multiplexed immune-modulating oncolytic virus.
- Engagement of tumor cells
The final step in the success of immunotherapy is T cell recognize, engage and lyse tumor cells. To avoid being identified, tumor cells down-regulate some components, including factors of antigen-forming and presentation pathways, such as TAP1, LMP2, LMP7, and tap-associated proteins. Tumor cells also down-regulate type 1 MHC through the loss of β2 tubulin or a single MHC allele. OV can reverse these effects. For example, by inducing type 1 interleukin, reovirus can increase the expression of type 1 and type 2 MHC in tumor cells and APCs. Even in antigen-presenting gene-deficient cells, OVs can increase the infiltration of NK cells and neutrophils, both of which can kill tumor cells by antigen and MHC mechanisms, respectively.
Outlooks
OVs and T cell immunotherapy
At present, there are many kinds of adoptive immune T cell therapy (ACT) products on the market. The treatment process is to extract T cells from the patient’s body for modification and amplification, and infuse to the patient’s body again. Poorly differentiated cells are optimal choice, but they often lead to terminal differentiation of T cells when expanded in vitro. There are teams studying ways to protect T cells from differentiation recently. However, a more realistic approach should be to find ways to overcome the various barriers that T cells encounter when they functioning. OVs can regulate the immune system and the tumor microenvironment, enabling T cells in ACT therapy to increase in value, continue to be transported in the body and play a role. In some preclinical and clinical studies, OVs combined with ACT only require fewer T cells to function more effectively. At the same time, OVs can not only be used as adjuvants, but also can lead to the release of TAAs, resulting in a more comprehensive T cell response.
Build better OVS
In order to better realize the above-mentioned potential OVs strategy, it is necessary to improve the system delivery capability of the current OVs and increase its spread and durability in the TME. On the other hand, a better understanding of our immune system’s response mechanisms to viral pathogens and tumors allows us to better design multiple anti-tumor OV vectors. In addition, we need to evaluate the optimal OV platform for achieving different immunotherapeutic effects. For example, in an oncolytic virus vaccine strategy, a virus with a simple genomic genome can be used as a pro-inflammatory virus, and it can quickly spread in TME and second lymphoid tissues and play a role; but conversely, if OV is used as a carrier to deliver immune molecules to TME, more complex and low level of replication virus is better. To better realize the latent potential function of OVs, OVs also need to be designed to regulate the spatiotemporal expression of transcribed genes. For example, ICIs, BiTEs, and MiTes can be expressed when a large number of OV infections and transmission processes are completed, thereby avoiding immature immune responses. We believe that with the development of genetic modification technology, the targeting and specificity of oncolytic viruses will be further enhanced, and the safety will be more assured, which will surely bring new hope for the treatment of tumors!
Reference
- Oncolytic viruses as engineering platforms for combination immunotherapy,Nature Reviews Cancer (2018)doi:10.1038/s41568-018-0009-4