CAR-T is chimeric antigen receptor T cell immunotherapy, which modifies T cell receptor by CAR and transforms it into the scFV segment in the binding domain of monoclonal antibodies. A classic CAR-T molecule generally consists of a single chain antibody (scFv) domain, cytoplasmic signaling domains and transmembrane domains. The modified CAR-T cells can specifically recognize tumor-associated antigens without restriction of MHC and optimize the targeting ability, killing activity and persistence of effector T cells.
Universal CAR T cells are generally engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, grate provides immune surveillance in case cancer cells exceptions again. But they uniquely recognize just CD19 – a problem, in that they kill even Normal B cells, so-called off-target.
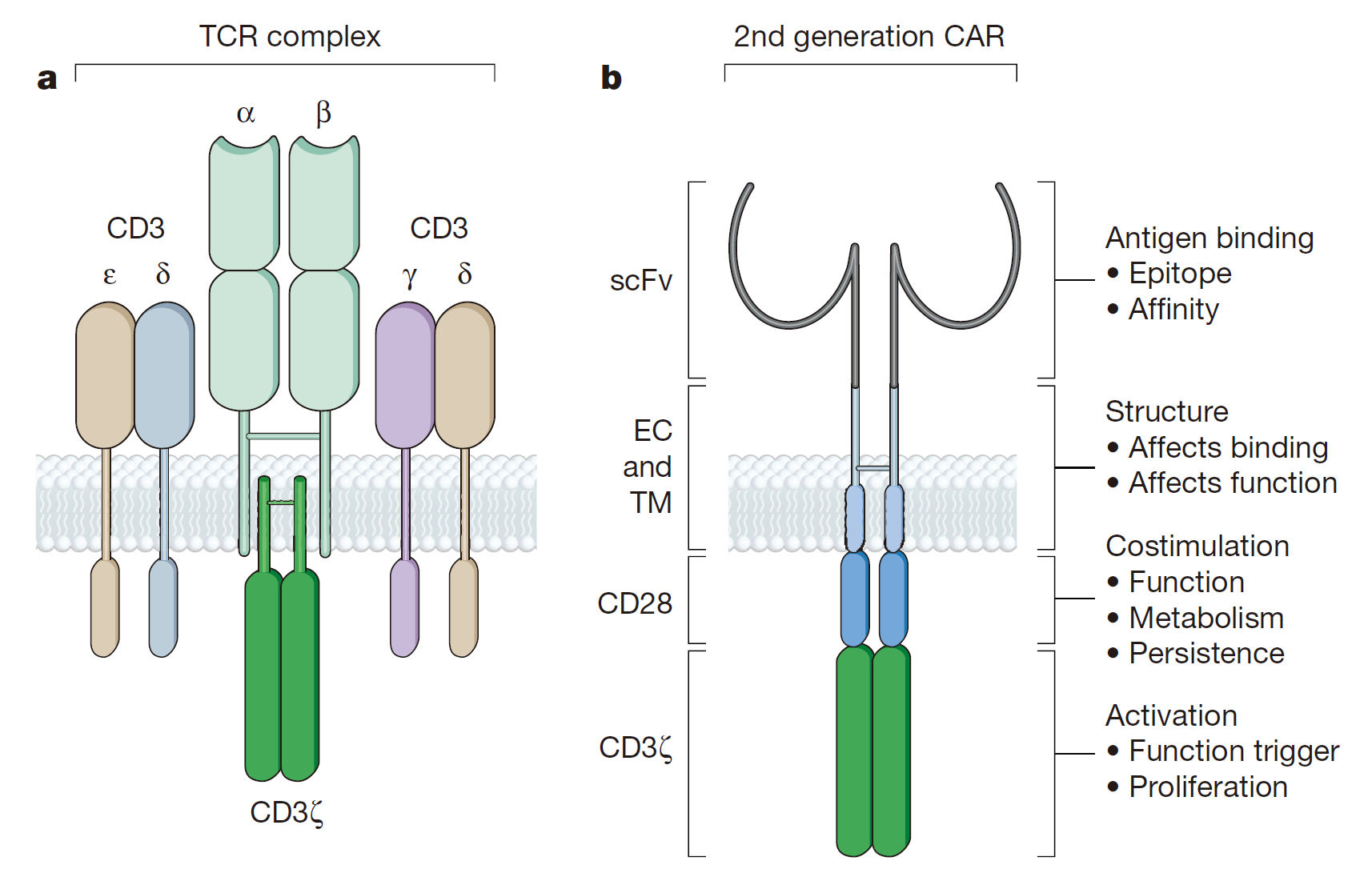
In 1989, the first generation of CAR was published in the Proceedings of the American Academy of Sciences (PNAS). The basic design includes a tumor-associated antigen-binding region, an extracellular hinge region, a transmembrane region, and a TCR complex ζ chain as an intracellular signal region.The first generation of CAR can only cause transient T cell proliferation and lower cytokine secretion, but can not provide long-term T cell expansion signal and sustained in vivo anti-tumor effect with the lack of co-stimulatory signal. CAR-T cells have been apoptotic before contacting with a large number of tumor cells. Julie combined CE7R single-chain antibody with CD3ζ for the cell adhesion molecule-L1 and constructed CAR vector by DNA of CAR plasmid with the introduction of “suicide gene” HyTK, treating recurrent neuroblastoma. Although there were no adverse effects associated with cell dose, each infusion of CAR-T cells did not survive for more than 1 week in vivo, and only one patient with the smallest tumor was partially relieved. In a word, the treatment effect was not satisfactory.
As for the second-generation of CAR gene construction, the second signal molecules (usually CD28, CD134 or CD137 ), the necessary signal for T cell survival, were assembled into T cells to increase the CAR-T immune memory effects and cytotoxicity on tumor cells.
The third-generation CAR-T structure not only includes “signal 1” and “signal 2”, but also contains additional co-stimulatory signals while CD28, CD134, CD137 molecules are assembled into CAR-T cells. Till constructed third-generation CAR-T cells (scFv CD20-CD28-CD137-CD3ζ) with retrovirus as a vector for the treatment of 3 patients with non-Hodgkin’s lymphoma. By PCR, the effector cells survived in vivo for more than 12 months.
CAR-T cells were introduced into cytokines (such as IL-12) in the 4th generation of CAR technology. When CAR-T cell receptor was used to recognize the target tumor, IL-12 was expressed at the tumor site, and all kinds of innate immune cells kill the tumor. The key point of CAR gene construction is to make a CAR T cell with a surface receptor binding to the dye fluorescein which is connected through a short linker to a molecule that binds specifically to a research expression of tumor body.
Reference
1. Sadelain, Michel, Isabelle Rivière, and Stanley Riddell. “Therapeutic T cell engineering.” Nature 545.7655 (2017): 423-431.