Regulatory T cells (Tregs) is a small group of immune cells, which specifically inhibit excessive immune activation and maintain immune homeostasis. In the past two decades, the advances in genome editing and chimeric antigen receptor (CAR) development have promoted the optimization of T cell therapy. These techniques are now used to enhance the specificity and function of Tregs, and autoimmune and transplant therapy based on Tregs is being developed rapidly.
-
The source and mechanism of Tregs
Regulatory T cells are a subset of T cells, which have the function of maintaining body balance and preventing autoimmunity. Tregs account for 5-10% of the total number of CD4+ T cells, which are characterized by the co-expression of CD4, CD25, FOXP3 and low-level CD127. A high level of FOXP3 and demethylation of specific demethylation region (TSDR) are prominent characteristics of Treg, and TSDR is a conserved region of FOXP3 gene.
Tregs can be divided into thymus-derived Tregs (tTregs) and peripheral Tregs (pTregs). During T cell development, primitive CD4+ T cells that receive intermediate T cell receptor (TCR) signals are driven to differentiate into Tregs. The difference in signal intensity determines whether primitive T cells differentiate into conventional T cells or Tregs. In addition, conventional T cells lacking FOXP3 gene will also be transformed into Tregs when they are repeatedly stimulated by non-autoantigens or exposed to IL-10 and TGF-β.
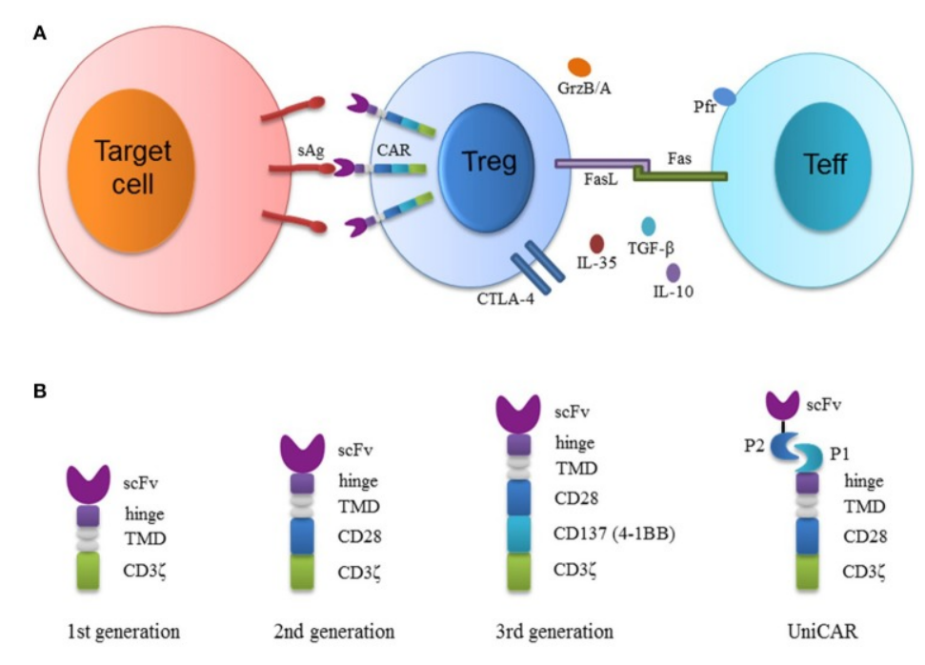
Treg suppresses the immune system through different mechanisms, including contact-dependent mechanisms, such as CTLA-4, and contact-independent mechanisms, such as the release of cytokines including IL-35 or IL-10. In view of their role in preventing autoimmune diseases, Tregs have an obvious prospect in promoting immune tolerance.
-
Adoptive Tregs: From polycloning to antigen specificity
The initial clinical trials of adoptive Tregs mainly used polyclonal or in vitro amplified Tregs. Although polyclonal Tregs have achieved encouraging results to some extent, the number of cells required for infusion is quite large. In addition, there is a risk of non-specific immunosuppression. In fact, virus reactivation after polyclonal Tregs infusion has been reported.
These shortcomings can be overcome by the use of antigen-specific Tregs. Compared with polyclonal Tregs, antigen-specific Tregs require fewer cells to perform more local and targeted inhibition. In addition, many studies have shown that in animal models, Tregs specific to the desired antigen are functionally superior to polyclonal or unmodified Tregs.
The traditional methods of producing antigen-specific Tregs mainly rely on antigen-presenting cells (APCs) and specific antigen amplification, or Treg engineered by TCRs. However, it is inefficient to amplify Tregs with APCs, while Tregs constructed with TCR (TCR-Tregs) are still limited by human leukocyte antigens (HLAs), which limits the modular application for different patients.
-
Engineered CAR-Treg
Another way to make Tregs specific is to use CAR to transduce these cells into CAR-Tregs. Compared with TCR-Treg, CAR-Treg has some unique advantages: (1) the targeting flexibility of CARs (any soluble or surface polyvalent antigen can be used as a target), (2) the activation of T cells expressing CARs bypassed the restriction of HLAs and increased the specificity through the activation of coreceptor signal.
In 2009, the first study to redirect human Tregs using CARs was carried out, and human CEA-CAR-Treg-mediated inhibition was observed in immunodeficient mouse models.
The most direct applications of CAR-Tregs are graft versus host disease (GvHD) and organ transplant rejection. Unlike most autoimmune diseases, there is a noticeably clear target in transplantation, that is, HLA molecules.
In 2016, HLA-A2 CAR-Tregs were first reported. Studies have shown that HLA-A2-CAR-Tregs inhibit the proliferation of Teff cells and block HLA-A2+ PBMC-mediated GvHD in immunodeficient NSG mice.
New applications of CAR-Tregs are still emerging, and B-cell-targeting antibody receptor (BAR) Tregs are the focus of research in recent years.
Human factor VIII (FVIII) injection is used to treat patients with hemophilia A, and over time, the development of anti-FVIII neutralizing antibodies will lead to an increase in morbidity and mortality. BAR with FVIII immunodominant domain (A2 or C2) as extracellular domain was designed to target FVIII-specific B cells, and the intracellular signal domain was still CD28-CD3 ζ. Strikingly, in in vitro study, human BAR-Tregs displaying A2 or C2 domains inhibited the production of anti-FVIII antibodies in mice immunized with recombinant FVIII.
-
Optimization of Engineered CAR-Treg
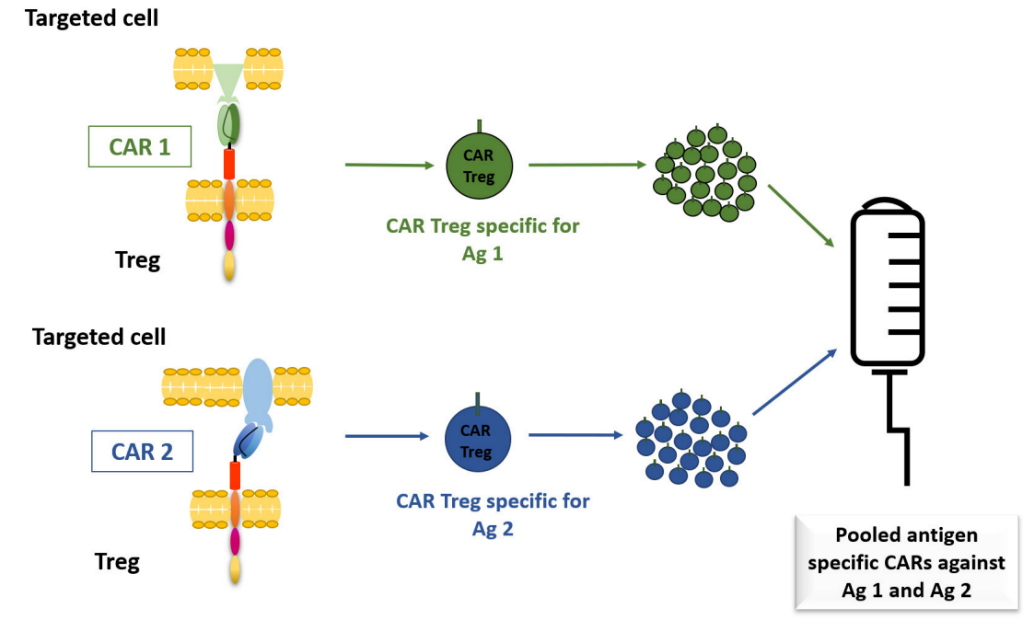
At present, most of the studies on preclinical disease models focus on monospecific CAR-Treg. Increasing the specificity of CAR-Treg can improve its therapeutic effect, and the functional advantages of Treg can be indirectly exerted through bystander inhibition.
The first option is the combination of CAR and Treg, which has been tested on CAR-T, including CD19 and CD123 for B-cell acute lymphoblastic leukemia (B-ALL), and HER2 and IL-13Rα2 for glioblastoma. However, this is logically challenging because the expansion of autologous CAT-Treg for different target antigens will be limited by the number of available autologous Tregs and highly expressed targeted antigens.
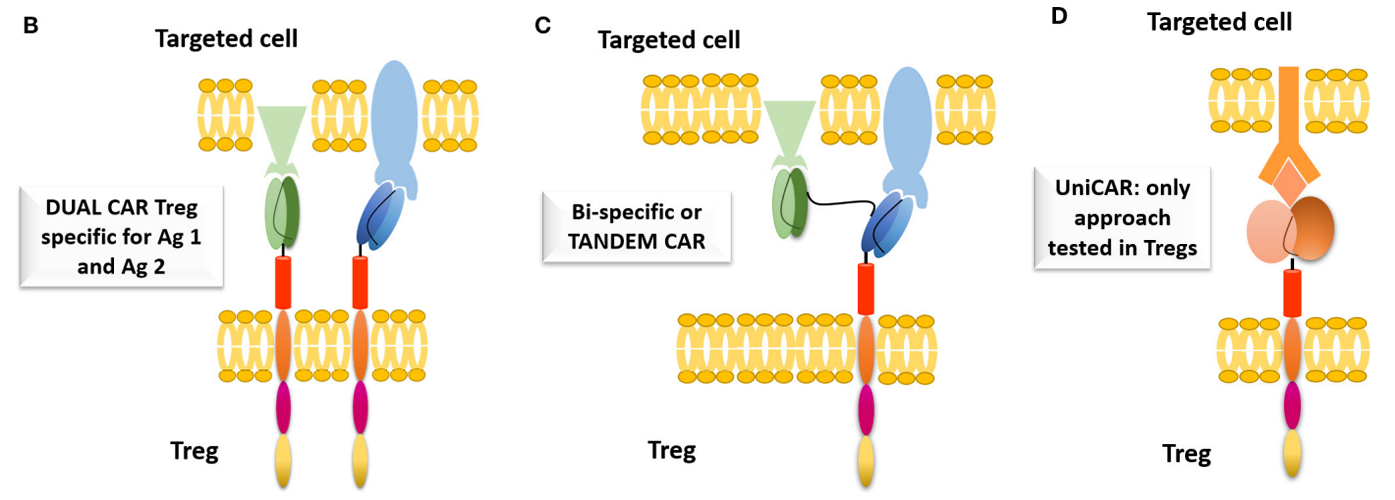
Therefore, Dual CAR-T cells were developed by using CAR-T cells transfected with two different specific antigens and signal domains. The combination of Dual CAR-T cells can prevent the escape of antigens more effectively than conventional CAR-T cells, showing the improvement of anti-tumor effect. In addition, bispecific CAR for two different antigens can also be used.
Developing modular or universal CAR (UniCAR) is another strategy, which allows custom control of Treg activities because the universal CAR is open to the target module, while the activation of universal CAR-Treg is strictly dependent on the target module.
-
Next-generation Treg engineering strategies
-
Synthetic biology
The development of Tregs as drugs for the treatment of autoimmune diseases is not limited to the application of TCRs and CARs. Synthetic immunology has produced many artificial receptors and systems, which are being tested in Tregs.
These systems include T cell antigen coupling agents that recruit endogenous TCR complexes to non-MHC targets, CARs that can be bound and activated by soluble ligands, and separable universal programmable CARs (SUPRAs).
-
Using cytokines
Cytokines play a key role in the immune response. CD25, the high affinity chain of IL-2 receptor, constitutively expressed in Tregs, can effectively deprive Teff cells. In addition, through the modification of engineered CAR-Tregs, proinflammatory cytokine signals can be converted into IL-2 or IL-10 signals, which will increase the inhibition of inflammation.
-
Gene editing
Preclinical studies on editing human T cell genes using CRISPR-Cas9 have been published, including knockout of CCR5 gene of CD4+ T cells to produce anti-HIV infection T cells; knockout of CD7 gene of CAR-T cells to prevent cannibalism; and knockout of PD-1 gene in anti-CD19 CAR-T cells to improve tumor clearance in humanized mouse models.
-
Upgrade the delivery system
At present, CAR-T cells are manufactured by transferring and integrating genetic materials into T cells with retroviruses and lentiviruses. However, these methods are time-consuming and expensive, and have safety problems.
Non-viral delivery methods are being developed, such as CRISPR-RNPs co-electroporation, which can knock DNA with more than 1 kb length into specific genomic sites of human T cells. This method is simple, safe, and the double-stranded DNA template is non-toxic. This method was used to correct the pathogenic mutation of CD25 gene of patients with CD25 mutation-caused autoimmune disease, which proved its potential application value. However, it was also limited by the size of the insertion fragment.
-
Design Tregs
One of the obstacles faced by Treg therapy is to ensure the survival of cells after infusion. Knockout of JNK1 gene in Tregs by gene editing can make these cells anti-apoptotic. JNK1-deficient Tregs can secrete higher levels of IL-10 and TGF β, which can protect the transplanted islets from rejection for 100 days.
In addition, Tregs may become unstable in an inflammatory environment and transform into pathogenic TH17 cells. By knocking out PRKCQ gene, the tendency of Tregs to differentiate into TH17 cells can be reduced, while the inhibitory function of Tregs can be maintained.
Finally, obtaining the latest generation of low-immunogenic pluripotent stem cells through gene editing, coupled with continuous efforts to differentiate stem cells into Tregs, may revolutionize the engineered Treg therapies.
-
Summary
At present, the use of Treg design to treat autoimmune diseases is in the ascendant, engineered CAR-Tregs show unique advantages in this aspect and have great potential. However, there are still many problems in the field of Treg biology and Treg therapy. But with the wide application of synthetic biology and gene editing techniques, continuous improvements in the design, manufacture, and application of Tregs are highly expected in the next decade.
References
1. Zhang, Qunfang, et al. “Chimeric antigen receptor (CAR) Treg: a promising approach to inducing immunological tolerance.” Frontiers in immunology 9 (2018): 2359.
2. Mohseni, Yasmin R., et al. “The future of regulatory T cell therapy: promises and challenges of implementing CAR technology.” Frontiers in immunology 11 (2020): 1608.