Exosomes have great therapeutic potential in cerebrovascular and neurodegenerative diseases; however, their poor natural targeting ability significantly reduces their efficacy. In contrast, engineered exosomes possess active targeting capabilities for specific cell types and tissues. Recently, scientists published a review in the journal Theranostics that summarizes the improved targeting functions of bioengineered exosomes, along with tracing and imaging techniques, delivery methods, blood-brain barrier (BBB) internalization, and the therapeutic effects of exosomes in cerebrovascular and neurodegenerative diseases. The review also evaluates the clinical opportunities and challenges within this research field.

Central nervous system diseases are complex and life-threatening, often leading to significant disability and mortality, and there are currently no ideal clinical treatments available. Stroke, a leading causes of permanent disability in adults, can be classified as either hemorrhagic stroke (~15%) or ischemic stroke (~80%). Therefore, actively exploring therapeutic methods to prevent stroke in high-risk populations and promoting neural recovery post-stroke are critical for improving outcomes for stroke patients. With global aging trends, developing potential new therapies for neurodegenerative diseases is also critical for improving the quality of life for the elderly and alleviating the burden on public health systems and caregivers.
Nanotechnology-based formulations have brought new hope for drug selection and targeted delivery in clinical practice, with the potential to overcome central nervous system barriers and transfer drug molecules to brain parenchyma. Traditional drug delivery systems (DDSs), including polymer nanoparticles (NPs), solid lipid nanoparticles, nanoemulsions, and liposomes, have proven effective for targeting brain regions. The development of cell-specific therapies has also opened new possibilities for treating neurological diseases; however, the use of live cells may induce autoimmune reactions or rejection, potentially increasing the risk of prolonged stimulation. Given the complexities of production, safety, and biocompatibility, natural nanovesicles present distinct advantages in this regard.
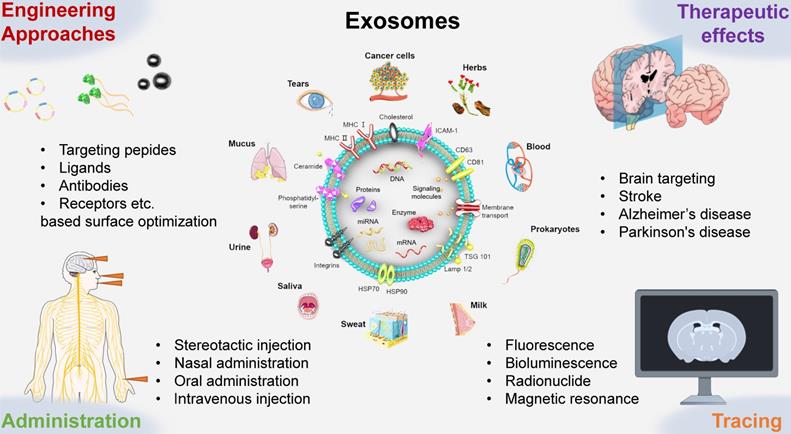
Exosomes were first discovered in reticulocytes through electron microscopy analysis in 1983. Since then, research has found that nearly all types of mammalian cells can secrete exosomes. Exosomes have been captured in primary cells from the immune and nervous systems, stem cells, various cancer cell lines, and bodily fluids such as blood, tears, urine, saliva, milk, and ascites. Moreover, exosomes have also been found in lower eukaryotes and prokaryotes. Recent studies indicate that plant-derived exosome-like nanoparticles (ELNs) can be extracted from plants like Rhodiola and dandelion. Despite over 30 years of research on exosomes, their biology and functions are not yet fully understood. As extracellular vesicles, exosomes are membrane-bound nanovesicles with diameters ranging from 40 to150 nm, containing proteins, RNA, DNA, lipids, metabolites, and cytosolic molecules. As such, exosomes perform a variety of functions, including facilitating intercellular communication, transferring antigens to dendritic cells (DCs), promoting angiogenesis, and enabling targeted homing. For example, the high expression of transmembrane proteins (particularly CD9, CD63, and CD81) and miRNAs like miR-124, miR-132, and miR-212 have been shown to possess exosome-targeting functions. In the nervous system, exosomes promote myelination, neurite outgrowth, neuronal survival, and tissue repair and regeneration. Exosomes have evolved from being considered simple “cellular waste bins” to playing key roles in many biological processes under both pathological and non-pathological conditions. Notably, exosomes from various sources can cross the BBB in different ways.
Given the structure and function of exosomes, they have become a popular drug in the treatment of cerebrovascular and neurodegenerative diseases. A previous study demonstrated that systemic administration of mesenchymal stem cell-derived exosomes (MSC-Exos) after a stroke improved functional recovery and enhanced angiogenesis, axonal remodeling, and neurogenesis. Another study suggested that MSC-Exos reduce inflammation and prevent aberrant neurogenesis by acting on astrocytes, showing great potential in treating neurological diseases such as stroke, temporal lobe epilepsy, Alzheimer’s disease, and Parkinson’s disease.
Despite the great potential of exosome homing in therapeutic applications, studies in animal models have confirmed that their targeting ability is relatively poor. As a result, extensive engineering techniques have been employed to modify exosomes and enhance their targeting capabilities. Another potential application of exosomes is in clinical monitoring. Due to their membrane structure being easily labeled and their close association with biomarkers, exosomes can be used to monitor changes in biomarkers during effective treatment. The specificity of exosomes enables them to have different half-lives and distribution patterns in the body, underscoring the important role of exosome biomarkers in tracking their fate. Furthermore, exosomes participate in the complex exchange of information between cells, regulating various processes (e.g., homeostasis, angiogenesis, and immune response) under both pathological and physiological conditions. Tracking exosomes in vivo can help us understand their mechanisms of action, biological functions, biodistribution, migratory abilities, and communication capacities. As exosomes become popular nanocarriers in drug delivery systems, the administration route is a critical aspect that cannot be overlooked.
In this review, researchers summarize exosome delivery routes, engineering strategies, targeting efficiency, therapeutic effects, and tracing methods. Additionally, the review discusses exosome internalization and specific regulatory roles in crossing the BBB. This review enhances our understanding of the biological significance of exosomes, promoting progress in the treatment of cerebrovascular and neurodegenerative diseases.
Reference:
Xu, Meng et al. “Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies.” Theranostics vol. 11,18 8926-8944. 18 Aug. 2021, doi:10.7150/thno.62330
Related Services:
Nanoparticle Tracking Analysis-based Exosome Characterization
