The development of effective vaccines for infectious diseases is one of the most successful global health interventions ever. Although subunit vaccines largely depend on the selection of antigens and adjuvants, the exposure modes and time scales of the immune system are often overlooked. Adjuvants play a crucial role in enhancing the quality and efficacy of immune responses, but unfortunately, poor control over the delivery of many adjuvants can limit their efficacy and lead to off-target toxicity. Therefore, there is an urgent need to improve adjuvant delivery technologies to improve the efficacy of adjuvants and enhance vaccine performance. The shape and size of nanoparticles mimic the structure of viruses, and so they have proven to be ideal carriers for improving antigen delivery, but exploration in adjuvant delivery is generally less common.
Recently, Professor Ben S. Ou and his team from the Department of Bioengineering at Stanford University developed a nanoparticle-based adjuvant construct to optimize CpG delivery, aiming to improve adjuvant efficacy. This research indicated that a single vaccination with CpG-NP hydrogels and soluble CpG-NP vaccines showed outstanding anti-spike protein antibody titers and broader antibody responses against immune evasion variants, reporting a simplified design of a widely implementable CpG-NP platform to increase efficacy and thus broaden and sustain the range of vaccines.
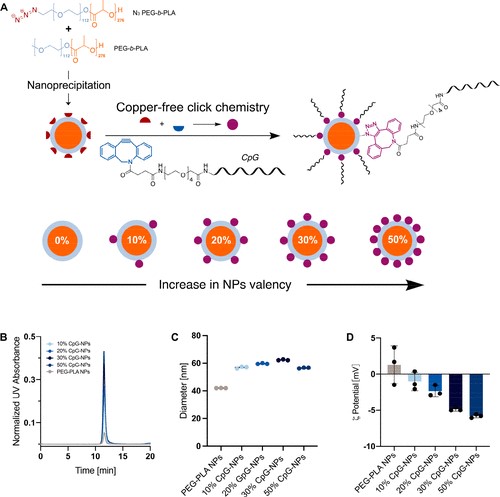
Fig.1. Design of CpG-functionalized NPs (Ou B S, 2024)
The results show that the presentation density of CpG has a great impact on the activation of TLR9, and in vitro intermediate densities of CpG on the NP surface (like 30% CpG-NPs) show maximum efficacy compared to soluble CpG. When these CpG-NPs are used as adjuvants for a COVID-19 candidate vaccine using SARS-CoV-2 spike protein, they can induce a better humoral response compared to soluble CpG adjuvants. In fact, compared to soluble CpG, vaccines composed of CpG-NP adjuvants can stimulate stronger and more sustained antibody titers, stronger recognition ability of related immune phagocytic variants, a balanced Th1 to Th2 response, and stronger neutralizing antibody responses. The impact on the rheological properties of embedding CpG-NP in a PNP hydrogel can be negligible compared to standard PNP hydrogel. Despite the different physicochemical properties of CpG-NPs and SARS-CoV-2 spike protein, immobilization in a hydrogel network results in similar diffusion characteristics, enabling the sustained co-delivery of both vaccine components. The humoral response generated by a single vaccination of CpG-NP hydrogel is equivalent to the primary enhancement scheme of soluble CpG-NP vaccines.
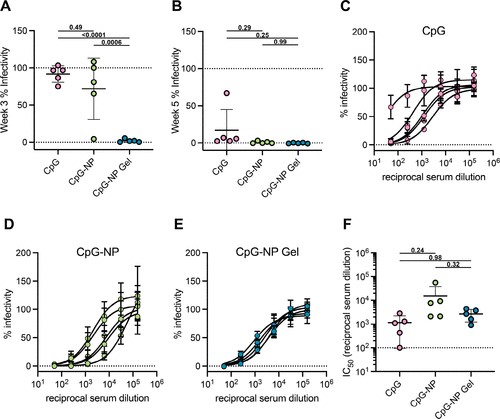
Fig.2. Single immunization of CpG-NP hydrogel elicits neutralizing antibodies in mice (Ou B S, 2024)
In conclusion, the good performance of a single immunization with the CpG-NP hydrogel vaccine can reduce the cost of clinical vaccination, improve patient compliance, ultimately resulting in faster vaccine acceptance and increased vaccination rates, all of which are key factors in addressing rapidly developing pandemics.
See Other Adjuvant Products:
| Aluminum | Oil Adjuvant |
| Saponin | Lipopolysaccharide |
| CpG | PRRs Agonist |
Reference:
Ou B S, Baillet J, Picece V C T M, et al. Nanoparticle-Conjugated Toll-Like Receptor 9 Agonists Improve the Potency, Durability, and Breadth of COVID-19 Vaccines[J]. ACS nano, 2024.
