At present, new antigen vaccines have become a hot direction in the field of cancer immunotherapy. With the release of some positive clinical data and more and more projects entering the clinical development stage, the anticipation for patent medicine of new antigen vaccines is increasing.
Professor Patrick A. Ott, a famous scientist in this field, and his colleague Eryn Blass published an article on Nature Reviews Clinical Oncology entitled “Advances in the development of personalized neoantigen-based therapeutic cancer vaccines“.
The article points out that individualized novel antigen vaccine gets benefit from the rapid development of sequencing and bioinformatics technology, having made positive progress in the past few years. Preliminary clinical results indicate that this novel vaccine shows strong tumor-specific immunogenicity and anti-tumor activity in patients with melanoma and other types of cancer.
In the review, Professor Ott summarizes the complex process of producing individualized novel antigen vaccines, reviews the types of T cells that can be induced by novel antigen vaccines, and outlines strategies that can enhance T cell response. In addition, the clinical research status of individualized novel antigen vaccine in cancer patients is discussed.
The following table summarizes several key clinical trials of individualized new antigen vaccines, including dendritic cells, peptides, and mRNA. The first trial to evaluate immunogenicity and immunological characteristics of individualized novel antigen vaccines (NCT00683670) used a strategy based on autologous dendritic cells. The results showed that the novel antigen vaccine can induce response of CD8+T cell and a variety of TCR, revealed that the therapeutic novel antigen vaccine could increase the diversity and breadth of T cell response for the first time.
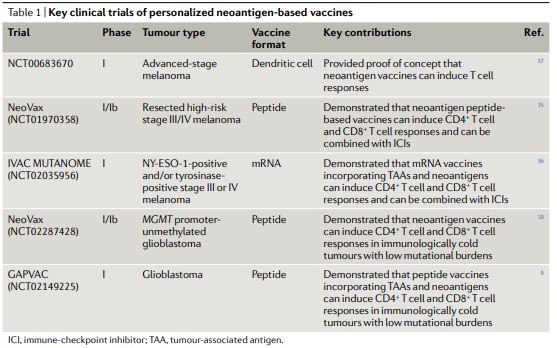
In a phase I trial of individualized novel antigen peptide vaccine NeoVax, specific CD4+ and CD8+T cells, which were not detected in previous studies, were induced after vaccination, in which CD4+T cells accounted for a large part of the response. Four stage III patients remained disease-free after vaccination when the median follow-up time was 25 months. Two stage IV patients developed relapse within a few months after the last vaccination, and then received treatment of pablizumab treatment. After that, the metastatic tumors of both patients completely disappeared and the anti-tumor T cell response expanded, indicating that the combination therapy has the potential to improve the vaccine-induced T cell response.
Another key phase I study investigated the mRNA vaccine (IVAC MUTANOME), which encodes melanoma antigens NY-ESO-1 and/or tyrosinase expressed by all patients, as well as individualized novel antigenic peptides. The trial (NCT02035956) was conducted in 13 patients with stage III or IV melanoma. The results showed that the mRNA vaccine fused with tumor associated antigen (TAA) and novel antigen could induce the response of CD4+T cells and CD8+T cells, and could also be combined with immune checkpoint inhibitors. Similar to the response observed in NeoVax study, the response of CD4+T cells induced by IVAC MUTANOME was stronger than that of CD8+T cells.
In addition to the melanoma targeted in the above three trials, the novel antigen vaccine has recently been tested in patients with glioblastoma. In a phase I/Ib trial (NCT02287428), 6 out of the 8 patients who received the NeoVax vaccine also received dexamethasone for brain edema, which hindered the immunogenicity of the vaccine. However, in other two patients who did not receive dexamethasone, the novel antigen specific CD4+T cells and CD8+T cells were detected in peripheral blood, and an increase in the number of T cells was observed in intracranial tumors after vaccination. Transcriptome analysis of tumor-associated T cells in one patient showed that CD4+ T cells and CD8+T cells showed cytotoxic characteristics, and the expression of inhibitory receptors (including TIM-3, LAG-3, TIGIT, and CTLA-4) also changed in varying degrees. These findings suggest that the novel antigen vaccine can also induce the response of CD4+T and CD8+T cells in immunological “cold” tumors.
These key tests provide important evidence for confirming the immunogenicity and therapeutic potential of individualized cancer vaccines based on novel antigens.
Scientists believe that the combined strategy of “new antigen vaccine plus immune checkpoint inhibitor” may be an effective means to improve the efficacy of new antigen vaccines.
