Respiratory syncytial virus (RSV) is the most common pathogen of viral pneumonia in children, which occurs mostly in newborns and infants less than 6 months old, as well as in immunodeficiency patients and the elderly. RSV can cause upper respiratory tract infection (such as cold-like symptoms) and lower respiratory tract infection (such as bronchiolitis and pneumonia). According to statistics, among children under 5 years old, more than 33 million cases of acute lower respiratory tract infection, more than 3 million visits, and more than 110,000 deaths are related to RSV infection. About 7.2 out of every 100,000 adults over the age of 65 die from RSV each year. RSV is a recognized global health problem, but no effective vaccine was developed in the past 50 years.
Nowadays, the research and development of RSV vaccine has achieved initial success. After RSV vaccines of Novavax and Pfizer enter phase III clinical trials, GlaxoSmithKline (GSK) recently announced that the phase III GRACE study (NCT04605159) to evaluate the safety and effectiveness of its respiratory syncytial virus vaccine candidate (GSK3888550A) for maternal immunization started to vaccinate subjects.
GRACE is a double-blind phase III study that will be conducted in 10,000 pregnant women aged 18-49 to evaluate the effectiveness of preventing neonatal RSV-related LRTI in healthy pregnant women who are immunized with a single dose of RSV maternal adjuvant-free vaccine intramuscularly. The study will also assess the safety of the vaccine in vaccinated mothers and their babies. The study is expected to be completed in early 2024, and interim results are expected to be released in the second half of 2022.
In October, GSK released data on the positive I/II safety, reactivity, and immunogenicity of its RSV vaccines for maternal immunization (GSK3888550A) and elderly immunization (GSK3844766A). Those are part of the GSK RSV vaccine project, which adopts different novel technologies to protect the most affected vulnerable groups, infants and the elderly, with three RSV candidate vaccines tailored for different populations (maternal vaccine, child vaccine, and vaccine for the elderly).
All the three vaccine candidates have obtained the fast-track qualification (FTD) of the FDA. Based on available data and contacts with regulators, phase III studies of RSV vaccine candidates for the elderly will be launched in the coming months, while phase I/II (RSV-negative infants) and phase II (RSV-seropositive infants) studies for children are still under way.
Tough Process of RSV Vaccine R&D
The development of the RSV vaccine started since the first discovery of the respiratory syncytial virus in humans in 1957. There was a major setback in the clinical trial of Pfizer’s formaldehyde inactivated RSV vaccine (FI-RSV) in the 1960s. More than 80% of the young children in the trial developed severe pneumonia after being infected with RSV, and two of them eventually died. Subsequently, the R&D of the RSV vaccine stepped into years of stagnation.
In the past decade, with the in-depth study of RSV etiology and structural biology and new research results in vaccine design and R&D, RSV vaccine research has made great progress.
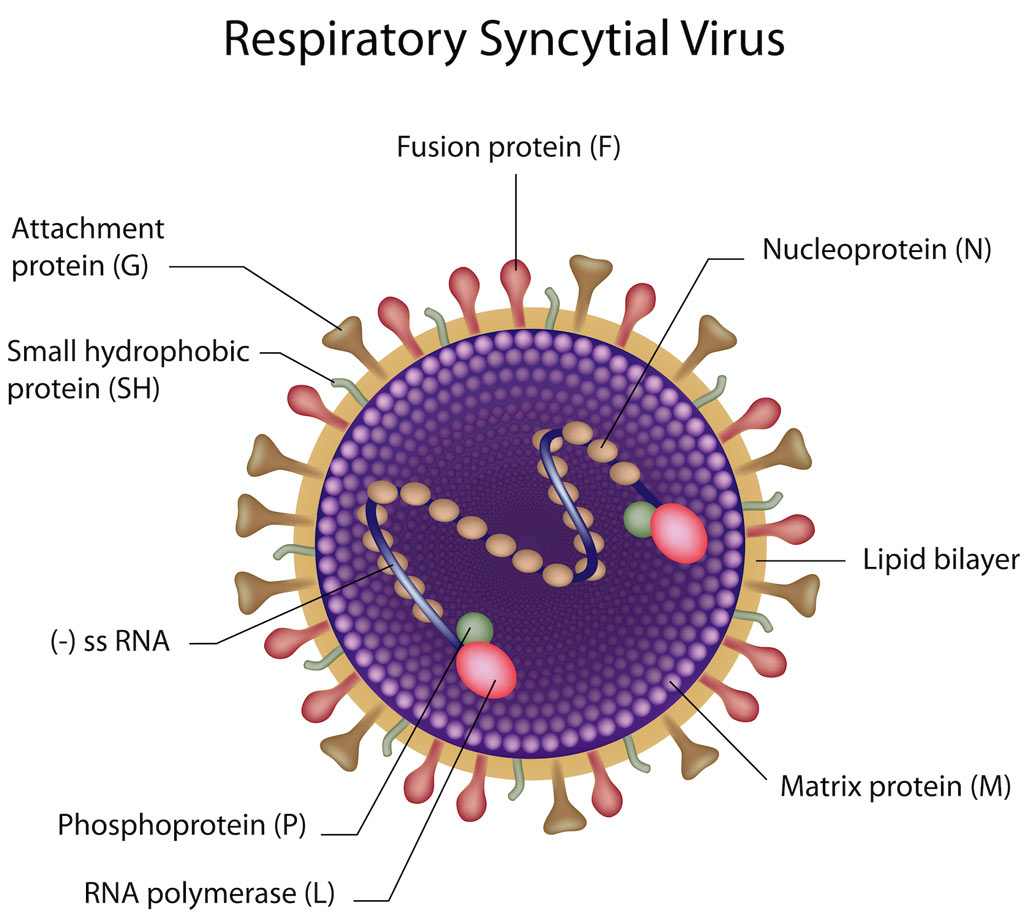
At present, the main antigen target of RSV vaccine development is F protein, which is one of the RSV envelope proteins and plays a pivotal role during virus infection. In addition to F protein, G protein is the only other target to neutralize virus surface. However, G protein has great variability among RSV strains, and its surface structure is little known, so it is not often used as vaccine antigen. Another possible vaccine target is the small hydrophobic protein (SH), containing a transmembrane domain and an extracellular domain, which plays a role in viral replication and inflammatory activation in vivo, and may induce antibody-dependent cell-mediated cytotoxicity (ADCC), so its extracellular domain is used as an antigen. Several other non-structural proteins (N protein, M2-1 protein, and M2-2 protein) of RSV are closely related to the induction of T cell immunity, so they are often used as targets of vaccine antigens alone or in combination with other antigens in vaccine design.
RSV Vaccine in Clinical Development
In recent years, a number of large pharmaceutical companies (such as GSK, Johnson, Pfizer, and Sanofi) and biotechnology companies joined the development of RSV vaccines and therapeutic drugs, and launched a number of clinical trials. Novavax, Pfizer, and GSK’s RSV vaccines have entered phase III clinical trials by now.
The RSVF protein recombinant nanoparticle vaccine (ResVax) developed by Novavax, is the first RSV vaccine to show effectiveness in phase III clinical trials. The phase II clinical trial in the third trimester of pregnant women and the healthy elderly shows that it has excellent safety, and immunogenicity, high tolerance, and good effect on virus prevention. However, phase III clinical trials in healthy pregnant women in the third trimester of pregnancy failed to reach the main endpoint. Although there were some problems in the application of ResVax in pregnant women, it would have great potential in the elderly and children.
In addition to ResVax, Pfizer’s vaccine candidate RSVpreF also started phase III clinical trials. In June of 2020, the NCT04424316 trial was conducted in pregnant women to evaluate the safety and effectiveness of the RSVpreF for maternal immunization to prevent lower respiratory disease in infants.
Last year, Janssen was awarded a Breakthrough Therapy designation by the FDA to prevent severe respiratory diseases caused by respiratory syncytial virus in people aged 60 and over.
In addition to the vaccine, Sanofi and AstraZeneca jointly developed the clinical phase III monoclonal antibody nirsevimab to prevent lower respiratory tract infections caused by RSV. The drug has been awarded Breakthrough Therapy designation by the FDA.
