In recent years, a new class of cancer therapies known as antibody-drug conjugates (ADCs) has been gaining significant traction. These innovative therapies represent a smarter, more targeted approach to cancer treatment, combining the precision of antibodies with the potency of chemotherapy. By acting as a sort of “guided missile” for cancer cells, ADCs are designed to deliver a powerful cytotoxic payload directly to a tumor, sparing healthy tissues from the harsh side effects of traditional chemotherapy.
The concept behind ADCs is brilliantly simple. A monoclonal antibody, which is a protein engineered to recognize and bind to a specific marker on the surface of cancer cells, is chemically linked to a potent, cell-killing drug, also known as a payload. Once the antibody finds its target on the tumor cell, the entire ADC-antigen complex is taken inside the cell through a process called receptor-mediated endocytosis. Inside the cell, the linker connecting the antibody and the drug breaks, releasing the cytotoxic payload to do its work, inducing cell death. This mechanism ensures that the drug is released exactly where it’s needed, maximizing its effectiveness while minimizing damage to healthy cells. This targeted delivery system has the potential to offer a wider range of therapeutic options and improve the efficacy of chemotherapy.
A Closer Look at the Components of an ADC
To truly appreciate the power of ADCs, it’s helpful to understand the three main components that make them so effective:
- The Monoclonal Antibody: This is the “homing device” of the ADC. The antibody is meticulously designed to latch onto a specific protein, or antigen, that is highly expressed on the surface of cancer cells. This specificity is what allows ADCs to differentiate between cancerous and healthy cells.
- The Cytotoxic Payload: This is the “lethal cargo” of the ADC. These drugs are extremely potent, far too toxic to be administered systemically on their own. The most common payloads are agents that interfere with cell division, such as microtubule inhibitors or DNA-damaging agents, which are highly effective at killing rapidly dividing cancer cells.
- The Linker: This is the crucial connection that holds the antibody and the payload together. The linker must be stable enough to keep the drug attached as the ADC travels through the bloodstream to the tumor, but also designed to cleave easily once inside the cancer cell. The stability of the linker is a critical factor in the safety and efficacy of an ADC, as premature release of the drug can lead to unwanted toxicity in normal tissues.
ADCs in Practice: A Focus on HER2-Positive Cancers
The success of ADCs has been particularly evident in the treatment of cancers that overexpress the human epidermal growth factor receptor 2, or HER2. HER2 is a protein that plays a role in cell growth and division, and its overexpression is a known characteristic of several aggressive cancers, most notably a subtype of breast cancer. Historically, this subtype of breast cancer has been associated with more aggressive features.
Two notable HER2-targeted ADCs have emerged as game-changers: one that combines a HER2-targeting antibody with a microtubule-inhibiting agent, and another that links the same antibody to a topoisomerase I inhibitor. These ADCs have demonstrated significant efficacy in treating HER2-positive breast cancers, offering a powerful alternative to traditional therapies.
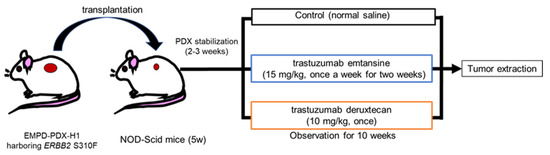
Fig.1 Treatment scheme for EMPD-PDX model mice using HER2-targeted ADCs.1
Expanding the Horizon: ADCs and Extramammary Paget’s Disease
While ADCs have shown great promise in breast cancer, their potential application is not limited to this area. Recent research has been exploring their effectiveness against other, rarer forms of cancer, such as extramammary Paget’s disease (EMPD). EMPD is a rare cutaneous adenocarcinoma that primarily affects the genital region in older adults. While the prognosis for early-stage EMPD is relatively good with surgical removal, the prognosis for advanced, metastatic EMPD is almost always poor. This highlights the urgent need for new and more effective therapeutic strategies.
Interestingly, EMPD shares some biological similarities with breast cancer, and studies have shown that HER2 is positively expressed in a significant percentage of EMPD cases, ranging from 15% to 60%. This makes HER2 a crucial therapeutic target for patients with HER2-positive EMPD.
A recent preclinical study investigated the efficacy of HER2-targeted ADCs against an EMPD model derived from a patient’s tumor, known as a patient-derived xenograft (PDX) model. This particular PDX model was chosen because it harbored a pathogenic mutation in the
ERBB2 gene, which encodes the HER2 protein. This mutation, known as S310F, has been shown to cause HER2 hyperactivation, a key mechanism that allows for the effective internalization of ADCs.
The results of this study were compelling. Mice with the EMPD-PDX tumors were treated with either of the two HER2-targeted ADCs, and both were found to significantly regress the tumors in just seven days. In fact, the tumors were no longer palpable after 14 days, suggesting they had been eradicated. Remarkably, there was no recurrence of the tumors for a period of 10 weeks.
Further analysis of the tumors after treatment provided more insight into how these ADCs work so effectively. Histopathological examination revealed a remarkably higher ratio of dead cells in the tumors treated with the ADCs compared to the control group. Additionally, the ratio of Ki-67-positive cells, a marker of cell proliferation, was significantly lower in the ADC-treated tumors. These findings suggest that the ADCs are highly effective at killing EMPD cells promptly.
These results are particularly noteworthy because they suggest that HER2-targeted ADCs could be a promising new treatment option for EMPD patients, especially in cases with the ERBB2 mutation or HER2 overexpression. This finding is consistent with previous research that showed the efficacy of HER2-targeted ADCs in other types of cancer models with similar ERBB2 mutations.
The Link Between HER2 and EMPD Progression
Beyond the promising therapeutic potential, the study also provided important insights into the role of HER2 in the progression of EMPD. The researchers examined tumor samples from 79 EMPD patients and found a significant correlation between positive HER2 expression and the presence of invasive lesions. This suggests that HER2-positive tumor cells may have an increased potential for invasion.
Furthermore, the study found that patients with positive HER2 immunostaining had a significantly worse disease-specific survival rate than those with negative HER2 staining. This indicates that HER2 status is a valuable prognostic marker and is associated with tumor progression in EMPD. The study also noted that disease-specific survival was significantly worse for patients with invasive EMPD and positive HER2 immunostaining compared to those with in situ cases and negative HER2 staining.
Future Directions and Limitations
While the findings from this study are very encouraging, it’s important to acknowledge some limitations. The study was preclinical and utilized only one specific EMPD-PDX model. To confirm these results and better understand the full potential of these therapies, it will be necessary to establish and test additional EMPD-PDX models and cell cultures.
Despite these limitations, the research suggests that HER2-targeted ADCs could be a novel and highly effective treatment for EMPD, particularly in cases with ERBB2 mutations or HER2 overexpression. The study highlights the importance of evaluating ERBB2 gene mutations in advanced EMPD to help guide treatment selection in the future.
The remarkable progress in ADC technology and its application to a wider range of cancers, from breast cancer to rare diseases like EMPD, signifies a major step forward in the field of oncology. By providing a more precise and potent way to combat cancer, ADCs offer new hope for patients with limited therapeutic options. This research is a testament to the power of targeted medicine and the ongoing efforts to develop smarter, more effective cancer treatments.
Reference
Tokuchi, Keiko, et al. “HER2-Targeted antibody–drug Conjugates Display Potent Antitumor activities in Preclinical Extramammary Paget’s Disease models: in vivo and immunohistochemical analyses.” Cancers 14.14 (2022): 3519. CC BY 4.0. https://doi.org/10.3390/cancers14143519