The concept of antibody–drug conjugates (ADCs) is based on exploiting the high specificity of a monoclonal antibody toward a selected tumor cell-surface antigen and enhancing the cell-killing capacity of the antibody by attaching a highly cytotoxic agent. An ADC can be divided into three main structural units (Fig. 1): the antibody (targets cancer marker); the linker (connect antibody and agent or drug); and the cytotoxic agent or drug (destroys target cells). To develop a successful antibody-drug conjugates (ADCs), every part needs to be selected carefully and many factors are taken into account. As common payloads of ADCs, MMAE (monomethyl auristatin E) and MMAF (monomethyl auristatin F) are both synthetic auristatin derivatives. Auristatin is a dolastatin 10-based auristatin analog. Dolastatin 10 was initially isolated from the sea hare Dolabella auricularia at a vanishingly low yield (~ 1 mg from each 100 kg). It is a unique linear pentapeptide comprising of several unusual amino acids (Fig. 2)
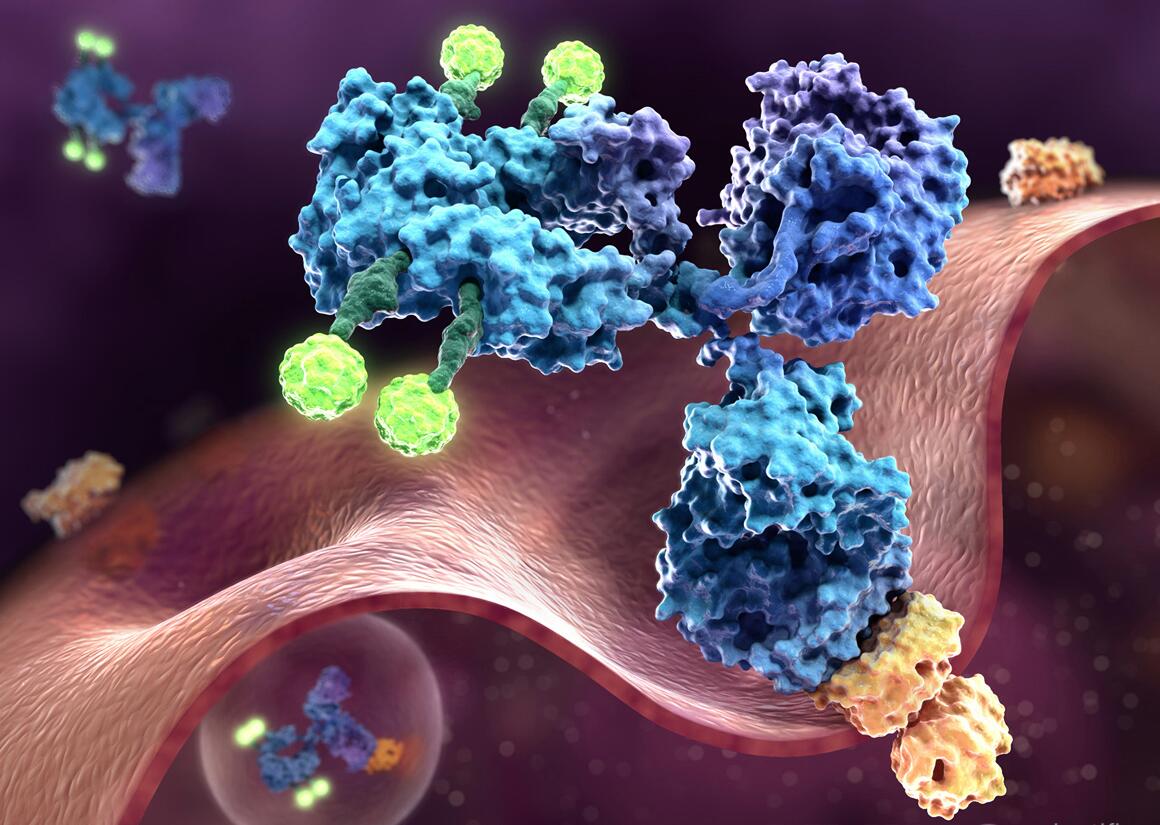 Fig 1 Antibody-drug Conjugates (ADCs)
Fig 1 Antibody-drug Conjugates (ADCs)
Both MMAE and MMAF are peptide analogs, which have limited impact on the physicochemical properties of the mAbs. MMAF differs from MMAE owing to a phenylalanine moiety at its C-terminus, contributing to its membrane impermeability. MMAE and MMAF are both highly stable molecules, showing no signs of degradation in plasma, human liver lysosomal extracts, or proteases such as cathepsin B. As free toxins, the cytotoxicity of MMAE and MMAF is about 200- and 1000-fold less potent than that of dolastatin 10 in lymphoma cells, respectively.
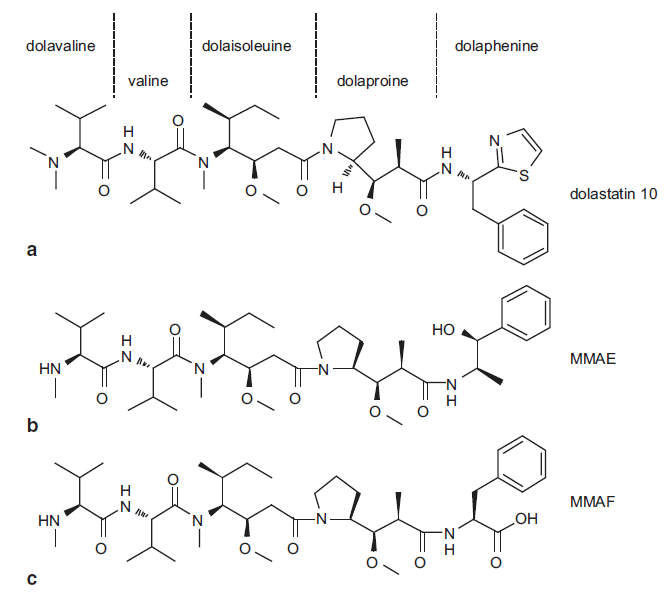 Fig. 2 Structures of dolastatin 10, MMAE, and MMAF. MMAE monomethyl auristatin-E, MMAF monomethyl auristatin-F
Fig. 2 Structures of dolastatin 10, MMAE, and MMAF. MMAE monomethyl auristatin-E, MMAF monomethyl auristatin-F
Mode of Action (MOA) – MMAE and MMAF
MMAE and MMAF function as mitotic inhibitors. MMAE is an antimitotic synthetic agent that inhibits cellular division via inhibition of tubulin polymerization. Monomethyl auristatin F (MMAF), which also inhibits cellular division via inhibition of tubulin polymerization, has attenuated activity compared to MMAE due to the presence of a charged C-terminal phenylalanine. Taking a typical auristatin-based ADCs – brentuximab vedotinas as an example, MOA of MMAE is showed as the figure 3. Learn more about MOA of auristatin.
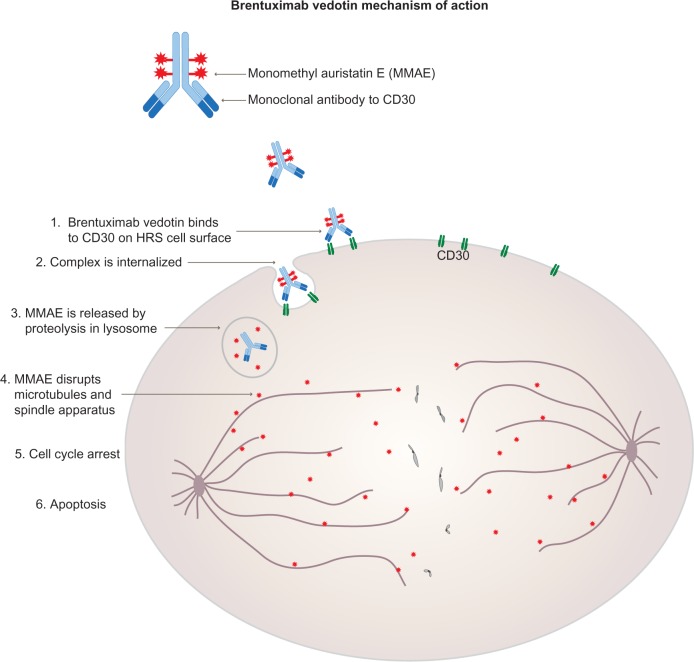 Fig. 3 Mechanism of action of brentuximab vedotin (Adcetris). Adcetris is approved by the FDA in August 2011 against Hodgkin and anaplastic large cell lymphomas
Fig. 3 Mechanism of action of brentuximab vedotin (Adcetris). Adcetris is approved by the FDA in August 2011 against Hodgkin and anaplastic large cell lymphomas
FDA-approved auristatin-based ADCs and Related Clinical Trials
Currently, there are four antibody-drug conjugates (ADCs) approved by US FDA: Mylotarg (2000, withdrawn in 2010), Adcetris (2011), Kadcyla (2013) and Besponsa (2017), while the second one Adcetris (the generic name is Brentuximab vedotin) is an auristatin-based ADCs. Adcetrisis formulated by the conjugation of brentuximab, an anti-CD30 (also known TNFRSF8) monoclonal antibody with payload monomethyl auristatinE (MMAE) via a cleavable peptide linker. CD30 is found on the surface of most classical Hodgkin lymphoma (HL) cells and in several types of non-Hodgkin lymphoma, but not commonly found on healthy cells. The auristatin-based ADC – Adcetris is approved in the United States 2011 for the treatment of Hodgkin lymphoma and one type of non-Hodgkin lymphoma: anaplastic large cell lymphoma (ALCL). More detailed description and international market of all these approved ADCs are elaborated in download resource.
Antibody-drug conjugates (ADCs) have become a powerful class of therapeutics in oncology. With four approved drugs on the market and a total of 95 ADCs in clinical trials, ADCs represent a growing class as the next generation for cancer treatment. According to the subcellular function of payloads (toxic agent or drug), they can be categorized into 4 major classes: tubulin inhibitors, DNA toxins, topoisomerase I inhibitors, and undisclosed. With the success of Adcetris, tubulin inhibitors such as auristatins (MMAE, MMAF) are the one major class of payloads used in ADCs in clinical studies. According to the survey from scientists in Creative Biolabs, tubulin inhibitors contribute to more than 30% of the payloads in clinical ADCs, which accounts for the largest proportion in all payloads (Fig. 4). It is concluded that auristatin has great potential for application in ADC discovery and development.
