The field of oncology has witnessed a paradigm shift in recent years with the rise of antibody-drug conjugates (ADCs). These sophisticated therapies combine the precision of monoclonal antibodies with the cytotoxic power of chemotherapeutic payloads, offering unprecedented opportunities to target tumors while sparing healthy tissues. As we navigate 2025, the ADC landscape is more dynamic than ever, with cutting-edge research pushing boundaries in target discovery, linker technology, combination strategies, and overcoming treatment resistance. In this blog, we delve into the latest advancements shaping the future of cancer care.
Target Expansion: Beyond Traditional Biomarkers
Historically, ADCs have focused on well-established targets like HER2 and TROP2. However, recent studies highlight a surge in novel target identification, driven by advancements in genomic profiling and computational biology.
Claudin 18.2: A Promising Target in Gastrointestinal Cancers
Claudin 18.2, a cell adhesion molecule overexpressed in gastric, pancreatic, and esophageal cancers, has emerged as a compelling target. Preclinical studies demonstrate that ADCs targeting Claudin 18.2 effectively deliver payloads to tumor cells, inducing apoptosis while minimizing off-target toxicity. Early-phase clinical trials report objective response rates (ORRs) exceeding 40% in heavily pretreated patients, with manageable safety profiles.
GPNMB: Addressing Resistance in Melanoma and Breast Cancer
Glycoprotein nonmetastatic B (GPNMB) is linked to tumor progression and resistance in melanoma and triple-negative breast cancer (TNBC). A newly developed ADC against GPNMB has shown remarkable activity in preclinical models, achieving complete tumor regression in murine studies. Phase I trials are underway to evaluate its efficacy in patients with refractory disease.
Integrin β4: A Novel Target for Solid Tumors
Integrin β4 (ITGB4), a transmembrane protein associated with tumor invasion and metastasis, is being explored as a target for ADC therapy. Preclinical data from a first-in-class ADC targeting ITGB4 reveal potent antitumor activity in non-small cell lung cancer (NSCLC), colorectal cancer, and head and neck squamous cell carcinoma models. The ADC efficiently internalizes into tumor cells, releasing cytotoxic payloads to disrupt microtubule dynamics and induce cell cycle arrest.
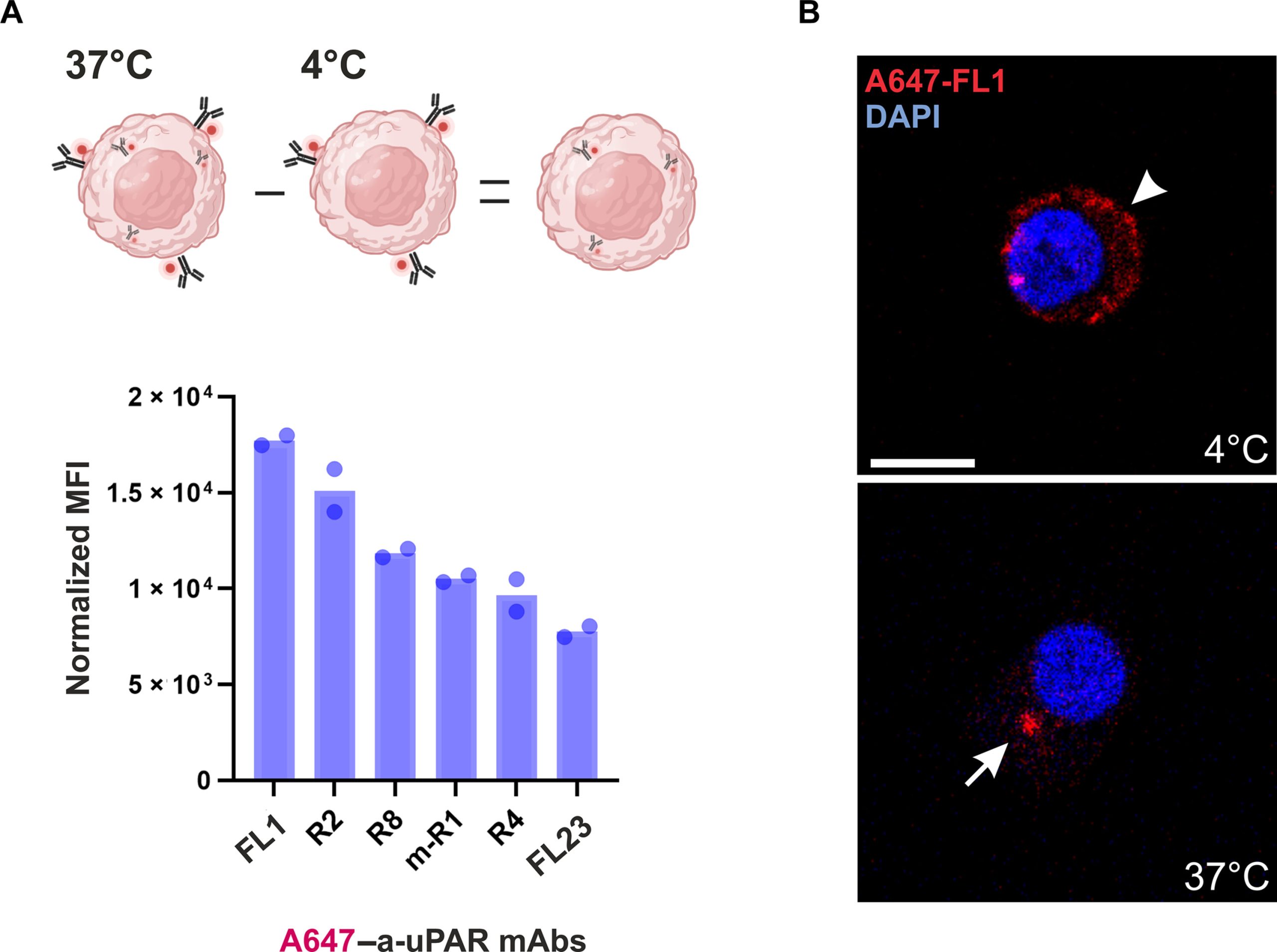
Fig1. Anti-uPAR mAb internalization in U937 cells.1
Linker and Payload Innovations: Enhancing Precision and Efficacy
The design of linkers and payloads is critical to ADC performance. Recent breakthroughs in these areas are optimizing drug delivery and overcoming treatment resistance.
pH-Responsive Linkers for Tumor-Specific Release
A novel class of pH-responsive linkers has been developed to enhance tumor selectivity. These linkers remain stable in systemic circulation but degrade under the acidic conditions of the tumor microenvironment, releasing payloads directly at the site of disease. Preclinical studies show that ADCs with pH-responsive linkers achieve higher tumor-to-normal tissue ratios compared to traditional cleavable linkers, reducing systemic toxicity.
Topoisomerase I Inhibitors: A Paradigm Shift in Payload Design
Topoisomerase I inhibitors, such as exatecan derivatives, have gained traction as payloads due to their ability to induce DNA damage and apoptosis. ADCs incorporating these payloads demonstrate broad-spectrum activity across solid tumors, including NSCLC and TNBC. Notably, a recent phase III trial reported a median overall survival (OS) of 12.88 months in patients with metastatic urothelial cancer treated with an ADC featuring a topoisomerase I inhibitor payload, significantly outperforming chemotherapy.
Bispecific ADCs: Hitting Two Targets Simultaneously
Bispecific ADCs, which target two distinct antigens on tumor cells, are emerging as a powerful strategy to enhance specificity and overcome resistance. For example, a dual-targeting ADC against EGFR and HER3 has shown impressive activity in preclinical models of NSCLC and nasopharyngeal carcinoma (NPC). In a phase I trial, this adc achieved an orr of 52.5% in EGFR-mutant NSCLC patients who had failed third-generation TKIs, with a disease control rate (DCR) of 87.5%.
Combination Therapies: Synergizing ADCs with Immunotherapy and Chemotherapy
The integration of ADCs with other treatment modalities is revolutionizing cancer care, particularly in challenging malignancies like TNBC and metastatic NSCLC.
ADCs + Immune Checkpoint Inhibitors
Combining ADCs with PD-1/PD-L1 inhibitors has yielded promising results. In a phase I/II trial of an anti-TROP2 ADC (SG) plus atezolizumab in PD-L1-positive TNBC, the combination achieved an ORR of 76.7%, with a median progression-free survival (PFS) of 12.2 months. The therapy also demonstrated activity in brain metastases, a common and aggressive complication of TNBC.
ADCs + Chemotherapy: Optimizing Sequencing and Dosing
Strategic use of ADCs alongside chemotherapy can enhance efficacy while reducing toxicity. A phase III trial evaluating an anti-TROP2 ADC (Dato-DXd) in metastatic NSCLC found that the ADC significantly improved PFS compared to docetaxel (4.4 vs. 3.7 months) with manageable myelosuppression. Subgroup analyses revealed prolonged OS in patients with high TROP2 expression.
ADCs + PARP Inhibitors: Targeting DNA Repair Pathways
Preclinical studies suggest that combining ADCs with PARP inhibitors may synergize in cancers with DNA repair deficiencies. A phase II trial of an anti-TF ADC (MRG004A) plus niraparib in BRCA-mutant breast cancer reported an ORR of 78.6%, with durable responses in patients with brain metastases.
Overcoming Resistance: Novel Strategies for Refractory Disease
Resistance to ADCs remains a significant challenge, but innovative approaches are emerging to address this issue.
Targeting Alternative Signaling Pathways
In HER2-positive breast cancer, resistance to trastuzumab-based ADCs often arises from bypass signaling through EGFR or HER3. A novel ADC targeting HER3 (Patritumab deruxtecan) has shown activity in preclinical models of HER2 TKI-resistant disease. In a phase II trial, this ADC achieved an ORR of 29.8% in EGFR-mutant NSCLC patients who had failed osimertinib and platinum-based chemotherapy.
Enhancing Tumor Penetration
To improve drug delivery to solid tumors, researchers are exploring ADCs with smaller antibody fragments or nanoparticles. A preclinical study demonstrated that a miniaturized ADC targeting EGFR achieved deeper tumor penetration and higher efficacy in murine models of pancreatic cancer compared to full-length antibody-based ADCs.
Personalized ADC Cocktails
Advances in liquid biopsy technology enable real-time monitoring of tumor mutations, allowing for personalized ADC selection. A pilot study in metastatic colorectal cancer showed that patients treated with ADCs matched to their tumor’s expressed antigens had significantly longer PFS compared to historical controls.
Safety and Tolerability: Managing Toxicities in the Clinic
While ADCs offer precise targeting, off-target toxicities remain a concern. Recent advancements in linker design and dose optimization are mitigating these risks.
Liver-Specific Payload Clearance
A novel ADC featuring a liver-clearing linker has been developed to reduce systemic exposure of payloads. Preclinical studies in non-human primates showed that this ADC effectively delivers payloads to tumors while minimizing hepatic accumulation, reducing the risk of hepatotoxicity.
Neurotoxicity Mitigation
Neurotoxicity, particularly with microtubule-disrupting payloads, is a common issue. A phase I trial of a novel tubulin inhibitor-based ADC reported minimal peripheral neuropathy, attributed to the use of a hydrophilic linker that limits penetration into the central nervous system.
Dose Escalation Strategies
Adaptive dose escalation protocols, guided by pharmacokinetic and pharmacodynamic biomarkers, are improving safety. For example, a phase I trial of an anti-Claudin 18.2 ADC used a Bayesian model to optimize dosing, achieving a 30% reduction in grade 3+ toxicities compared to traditional 3+3 designs.
The Future of ADCs: Emerging Technologies and Unmet Needs
Looking ahead, the ADC field is poised for further innovation, with several exciting developments on the horizon.
CRISPR-Guided ADC Development
CRISPR-Cas9 technology is being used to screen for novel ADC targets and optimize payload efficacy. A recent study identified CD44v6 as a potential target for ADC therapy in gastric cancer using CRISPR-based loss-of-function screening.
AI-Driven Design
Artificial intelligence (AI) is accelerating ADC discovery by predicting optimal antibody-linker-payload combinations. A machine learning model developed by a research consortium accurately predicted ADC stability and efficacy in silico, reducing preclinical development timelines by 40%.
ADC Vaccines
Researchers are exploring ADCs as immunotherapeutic agents by conjugating antigens to payloads. A preclinical study demonstrated that an ADC targeting HER2 conjugated to a tumor-associated antigen induced a robust T-cell response, leading to long-term tumor immunity.
The ADC revolution is transforming cancer treatment, offering unprecedented precision and efficacy. From novel targets and linker technologies to combination strategies and AI-driven design, the field continues to evolve at an astounding pace. As we look to the future, ADCs hold immense promise to address unmet medical needs and improve outcomes for patients with even the most aggressive malignancies. Stay tuned for updates as we continue to unravel the next chapter in this exciting journey.
Creative Biolabs offers end-to-end ADC development services, covering antibody discovery to preclinical evaluation. Leveraging expertise in antibody engineering and linker-drug design, the company supports development of specific, potent ADCs for targeted cancer therapy.
- One-Stop ADC Development Services: Covers the full ADC pipeline: tumor antigen ID, antibody discovery, linker-drug design, conjugate synthesis, and in vitro/in vivo evaluation.
- Tumor Surface Protein Specific Antibody Discovery Services: Isolates monoclonal antibodies targeting tumor cell-surface proteins for high-affinity, specific ADC targeting.
- Tumor Microenvironment Antigen Specific Antibody Discovery Services: Discovers antibodies against tumor microenvironment antigens (e.g., stromal proteins) to enhance ADC efficacy and reduce toxicity.
References:
Metrangolo, Virginia, et al. “Targeting uPAR with an antibody-drug conjugate suppresses tumor growth and reshapes the immune landscape in pancreatic cancer models.” Science Advances 11.3 (2025): eadq0513. https://doi.org/10.1126/sciadv.adq0513