At present, the vaccine research for the Coronavirus Disease (COVID-19) is in progress, but the drug research and development for severe COVID-19 remains a thorny scientific problem. Up to now, there is no specific drug for patients with severe COVID-19. Current studies have shown that there are neutralizing antibodies to SARS-COV-2 in patients with severe COVID-19, showing the excessive release of pro-inflammatory factors, suggesting immune dysfunction in patients. At present, the antibody therapy represented by plasma therapy has not shown a positive therapeutic effect in randomized controlled trials, and thus its therapeutic value needs to be further clarified. Therefore, the development of drugs and treatments for severe COVID-19 patients poses a serious challenge to the global scientific community.
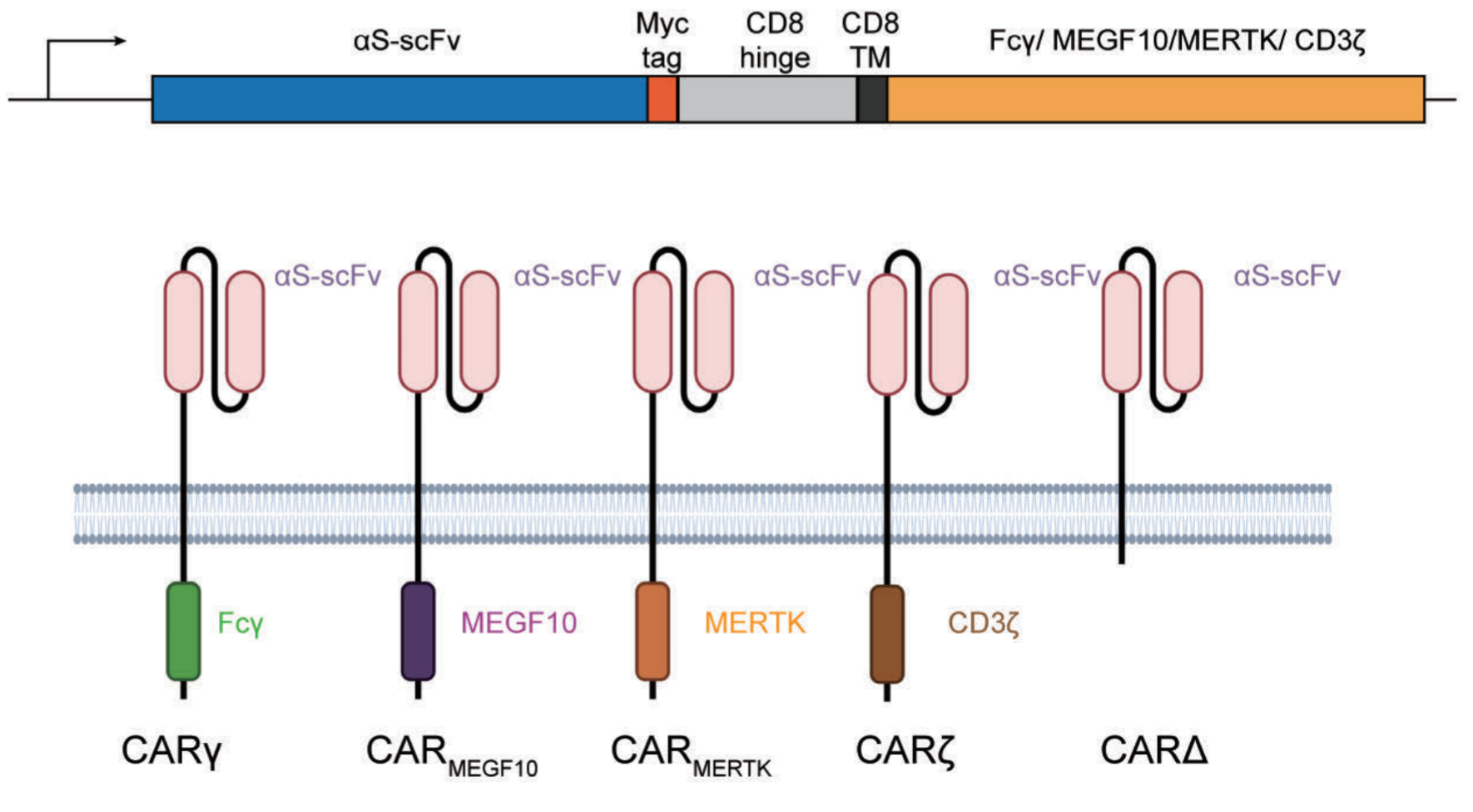
Recently, the Shi Hu team of the College of Basic Medicine Studies of the Second Military Medical University, China, publicly submitted the latest research manuscript entitled “CAR Macrophages for SARS-CoV-2 Immunotherapy” on the bioRxiv preprint platform. The manuscript of this study discloses the design, preparation and characterization of chimeric antigen receptor (CAR) macrophages (CAR-Macrophage) for SARS-COV-2 virus clearance. In this study, in addition to the common CAR design based on CD3 and Fc receptors, the research team cleverly constructed a way to construct the intracellular signal domain of chimeric antigen receptors (silent CARs) using macrophage-specific receptors (TAM family receptor member MERTK) on the basis of CAR receptor design.
CAR is a synthetic receptor that redirects T cell activity to a specific target. In the extracellular domain, CAR constructors include single-stranded variable (scFv) or antigen recognition domains in the form of binding receptors/ligands, transmembrane domains that provide scaffolds and signal transduction, as well as intracellular domains from T cell receptors (TCR) and costimulatory molecules that stimulate T cell activation. Based on the long-standing interest in using macrophages to combat tumor growth, engineered human macrophages using CAR have been developed and their anti-tumor potential has been characterized. Macrophages are key effectors of the innate immune system, which are responsible for sensing and responding to microbial threats and promoting tissue repair. Therefore, the authors hypothesized that CAR macrophages could be used to fight against SARS-CoV-2. However, it has been found that the highly inflammatory macrophage response is harmful to the host, especially in severe infections (including SARS-CoV-2) and cytokine release syndrome (CRS), which is also the most important complication associated with CAR-T cell therapy, raising questions about the safety of using CAR macrophages to clear the virus.
Based on the recognition of S protein, the authors developed a series of CARs and tested their ability to induce phagocytosis of SARS-CoV-2 virions. They reported that compared with other CAR, a CAR with the intracellular domain of MERTK of the TAM receptor family did not show significant killing effect in the antigen-based cell model, but did show antigen-specific clearance of SARS-CoV-2 virions without secretion of pro-inflammatory cytokines in vitro.
In summary, the data show that the synthesis method based on CAR is suitable for the treatment of COVID-19. In addition to direct viral clearance by CAR macrophages, the authors also found evidence that MERTK-based CARs do not induce further upregulation of inflammatory cytokines, thus increasing the possibility that CAR macrophages can be used as an effective therapeutic agent in patients with severe COVID-19.
Reference:
Fu, Wenyan, et al. “CAR Macrophages for SARS-CoV-2 Immunotherapy.” bioRxiv (2020).