As a precision targeted therapy for tumor treatment, CAR-T (chimeric antigen receptor t-cell immunotherapy) has achieved promising results in clinical tumor treatment with optimization and improvement in recent years. It is cutting-edge tumor immunotherapy that is accurate, fast, efficient, and has the potential to cure cancer.
However, although 3 CD19-targeted CAR-T therapies, including Kymriah, Yescarta, and Tecartus, have been approved for clinical use, most are still in clinical trials. Based on the current clinical manifestations of CAR-T, the existing therapies have three major pain points. The first is the high cost; secondly, the safety remains a challenge for certain indications; thirdly, patients with these therapies are prone to relapse after treatment.
So, does CAR-T therapies have the possibility to be upgraded in the next generation?
- The First Road for Upgrading: Non-viral Vector Delivery System
Non-viral vector technology has a long history, and at present, there are several non-viral vectors for CAR-T cell preparation at home and abroad. The piggyBac non-viral vector (PB transposon) represented by Poseida/Shanghai Cell Therapy Group, Sleeping Beauty non-viral vector (SB transposon) represented by Ziopharm/Precigen and mRNA vector represented by the Carl June’s team at the University of Pennsylvania.
| Viral vector (Lentivirus/Retrovirus) | PB transposon | SB transposon | mRNA | |
| Representative | Novartis/Kite | Poseida | Ziopharma | Carl June’s team |
| Vector | Lentivirus/Retrovirus | Plasmid/Plasmid+mRNA | Plasmid/plasmid+mRNA | mRNA |
| Integration method | Random insertion in the genome | Random insertion in the genome | Random insertion in the genome | No insertion in the genome |
| Delivery method | Centrifugal transfection | Electroporation | Electroporation | Electroporation |
| Status | Approved | Phase II | Phase I | Phase II |
CAR-T cell therapy products produced by non-viral vectors is simpler in the production process and also easier to perform quality control comparing with that of viral vectors. At the same time, in terms of storage, the plasmid DNA or mRNA used by the non-viral vector has higher stability, lower storage requirements and longer validity period than the RNA virus of the viral vector system. The advantage of simplified production and lower cost is prominent since CAR-T cells prepared by viral vectors are expensive involving numerous production and quality controls.
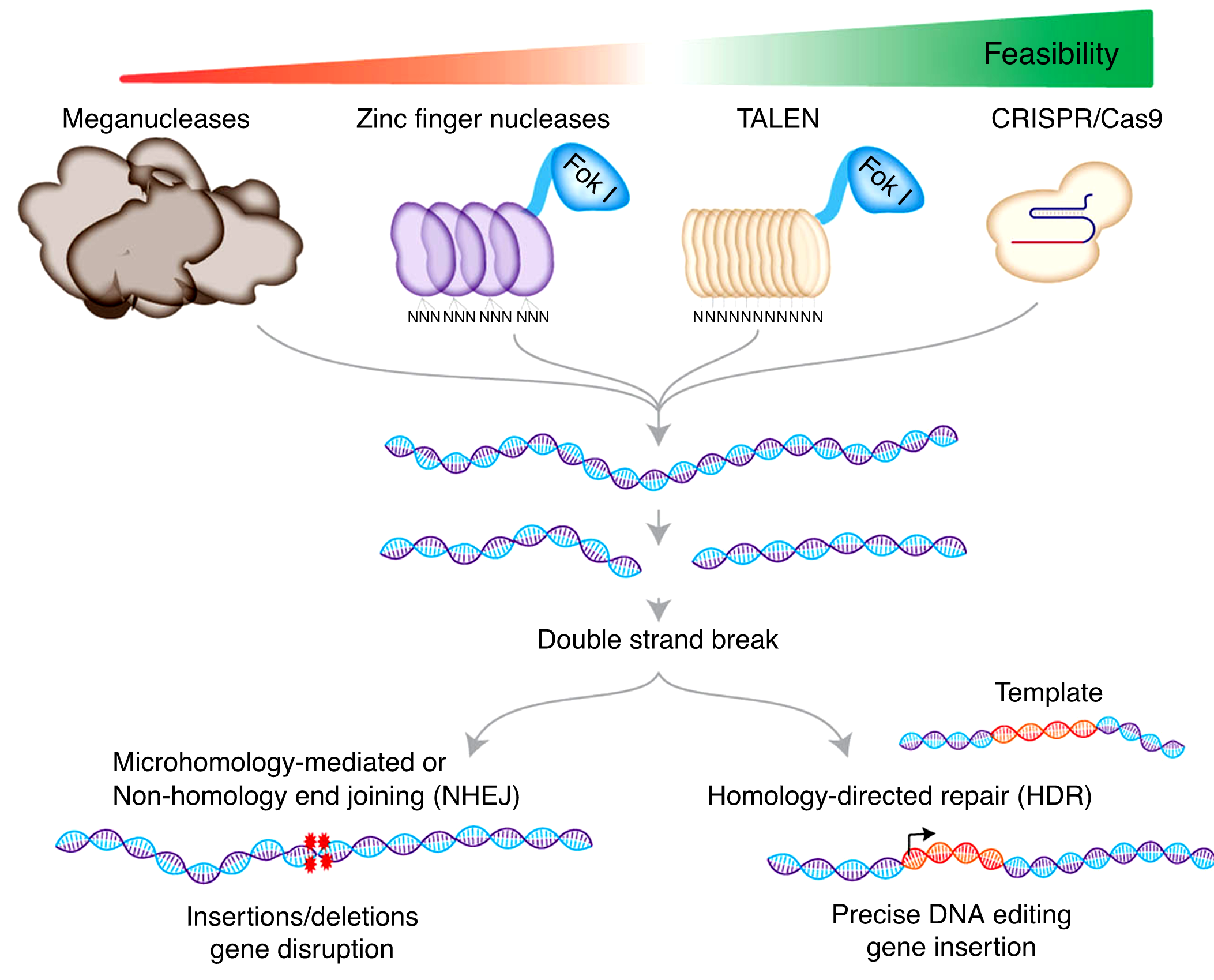
- The Second Road for Upgrading: Gene Knock-in Technology
The gene knock-in technology in CAR-T has been used only in the past two or three years. At present, the technology is mainly mastered by several gene editing technology companies. The representatives to achieve efficient gene insertion are ZFN combined with adeno-associated virus (AAV) technology of Sangamo Therapeutics, ARCUS gene editing technology combined with AAV technology of Precision Bioscience, CRISPR-Cas9 gene editing technology to combine with AAV technology of Fate Therapeutics, and megaTAL gene editing technology combined with AAV technology of bluebird bio. The comparison of various technologies is as follows:
| ZFN | ARCUS | CRISPR-Cas9 | megaTAL | |
| Representative | Sangamo Therapeutics | Precision Bioscience | Fate Therapeutics | bluebird bio |
| Vector | mRNA+AAV | mRNA+AAV | mRNA+AAV | mRNA+AAV |
| Locus | TRAC | TRAC | TRAC | TRAC |
| Status | Preclinical | Phase I | IND Application | Unknown |
Gene knock-in technology is currently mainly used in general-purpose CAR-T in the field of cell therapy, which can achieve CAR gene knock-in and TCR gene knock-out in a one-step method. In addition, gene knock-in technology can theoretically improve the effectiveness of CAR-T therapy. There is evidence that the integration of the CAR gene into the TRAC locus can greatly improve the efficacy of CAR-T therapy in mouse models. Carl June’s team also found that the random insertion of TET2 genetic locus into the CAR gene can give patients long-term benefits. What’s more, the fixed-point insertion technology can avoid the risk of cancer caused by random insertion.
Reference
Longchang, X. I., and G. E. Zhishen. “Nonviral CRISPR/Cas9 delivery systems for gene editing.” Journal of Functional Polymers 32.5 (2019): 582-592.