Alzheimer’s disease (AD) is A chronic neurodegenerative disease characterized by cognitive impairment and multiple pathological changes, including beta-amyloid protein (Aβ) deposition, Tau protein aggregation, altered immune response, and brain atrophy. Despite ongoing research into its underlying pathogenesis, the cause of AD remains unclear. Diagnostic criteria for AD do not effectively identify preclinical patients, and current AD treatments do not delay or reverse the progression of the disease. Since Non-Human primates (NHP) are closely phylogenetic related to humans, have similar neuroanatomy, comparable genetic regularity, and highly complex similar higher-order cognitive functions, making them better models of AD than rodents, Therefore, there is an urgent need to develop non-human primate (NHP) models to facilitate AD disease research.
A review titled “Non-human Primate Models of Alzheimer’s Disease” was recently published in the Journal of Exploratory Research in Pharmacology, a journal of Huayu Publishing House systematically compared AD-related pathological features and behavioral changes between spontaneous (natural aging) and (artificially) induced NHP AD models. In recent decades, both spontaneous and induced NHP AD models have been used to facilitate the development of neuroimaging trackers and therapeutics, helping to translate laboratory findings into clinical trials involving humans. By developing guidelines regarding NHP species, type and dose of inducer, injection frequency, and vaccination time, it is expected to establish a standardized NHP AD model. Further development of NHP can be facilitated through a comprehensive evaluation of NHP, including all AD-related conditions and an extensive behavioral examination. The NHP AD model has made important contributions to AD research and is expected to better reproduce the characteristics of AD and present greater translational potential in the near future.
AD animal model
Despite over 100 years of extensive research on AD, the underlying pathophysiological mechanisms remain unknown, the etiology of the disease remains incompletely understood, accurate diagnostic tools are not widely available for population screening, and there is a lack of disease-modifying treatments (DMTs). Given these urgent needs in the field, it has become critical to develop and study reliable animal models of AD to enable the study of preclinical and prodromal stages of AD, which are difficult to obtain in AD patients. This review evaluates as comprehensively as possible the different model animals in AD-related research. Among them, various rodent models of AD have occupied the mainstream in the field in recent decades. Mutations in genes associated with Aβ and Tau provided a solid basis for the generation of hundreds of transgenic mouse models (Figure 1), which have been developed over time to better mimic the specific features of AD and facilitate experiments. Translational applications of chamber discoveries. Rodent models have many advantages, including low cost, large sample sizes, easier genetic manipulation, and routine animal care. Unfortunately, because the complexity of rodent brain structures and neural circuits is not comparable to humans, their translational potential for the development of diagnostic markers and therapeutics is also low.
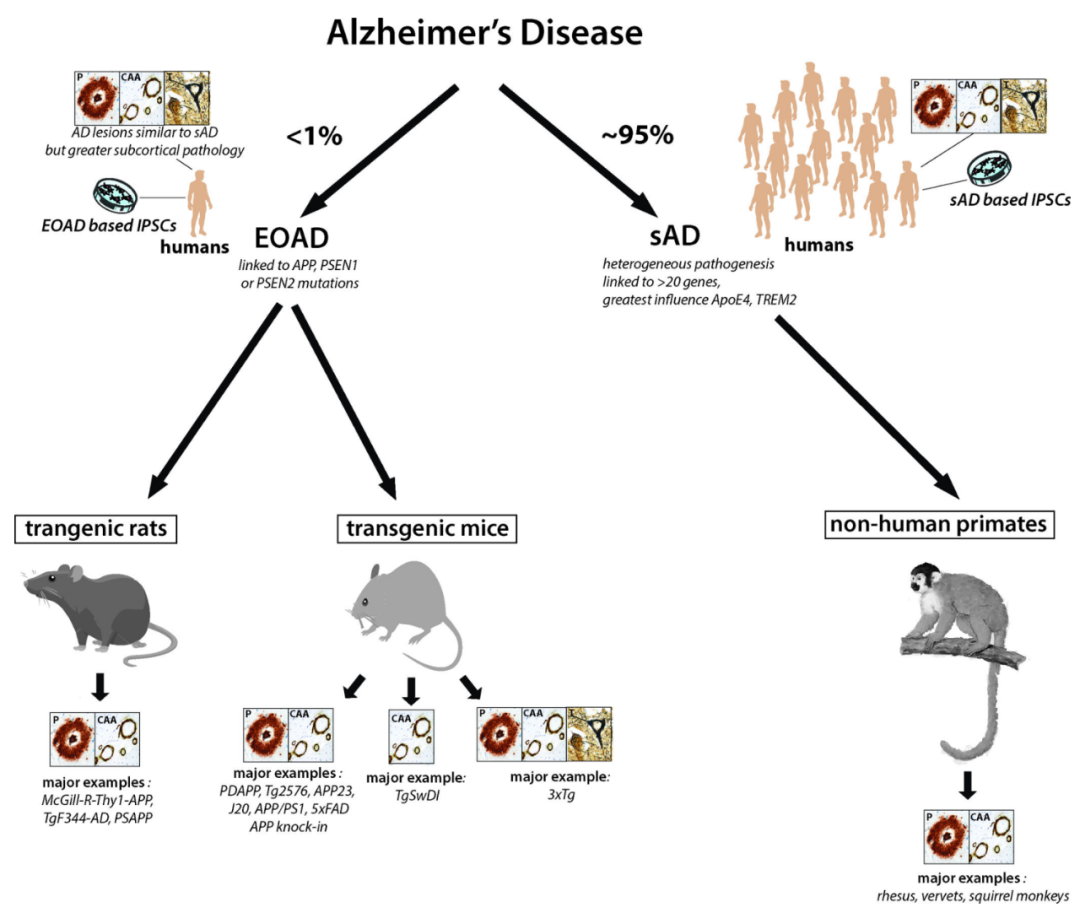
Fig.1 Schematic diagram of the main animal models for Alzheimer’s disease (AD) research.
Less than 1% of AD cases are cases of early-onset familial Alzheimer’s disease (EOAD) caused by autosomal dominant mutations in APP, PSEN1, or PSEN2. However, all major transgenic rodent models express these variant forms of APP and PS1. Currently, the best animal model of sporadic AD (sAD) is non-human primates (NPHs). Types of neuropathology consistently present in each model are shown in boxes; P: plaque; CAA; congestive amyloid angiopathy; T: neurofibrillary tangles. The presence of preplaque lesions in these animal models is not sufficient to indicate the presence of neurofibrillary tangle lesions. Therefore, only 3xTg mice express all 3 pathological hallmarks of AD.
Therefore, to understand complex age-related human diseases, the use of nonhuman primate (NHP) brains is highly desirable because of its closer phylogenetic relationship with humans, similar neuroanatomical structures, and Similar genetic characteristics, similar complex neural circuits and higher-order cognitive functions (Table 1).
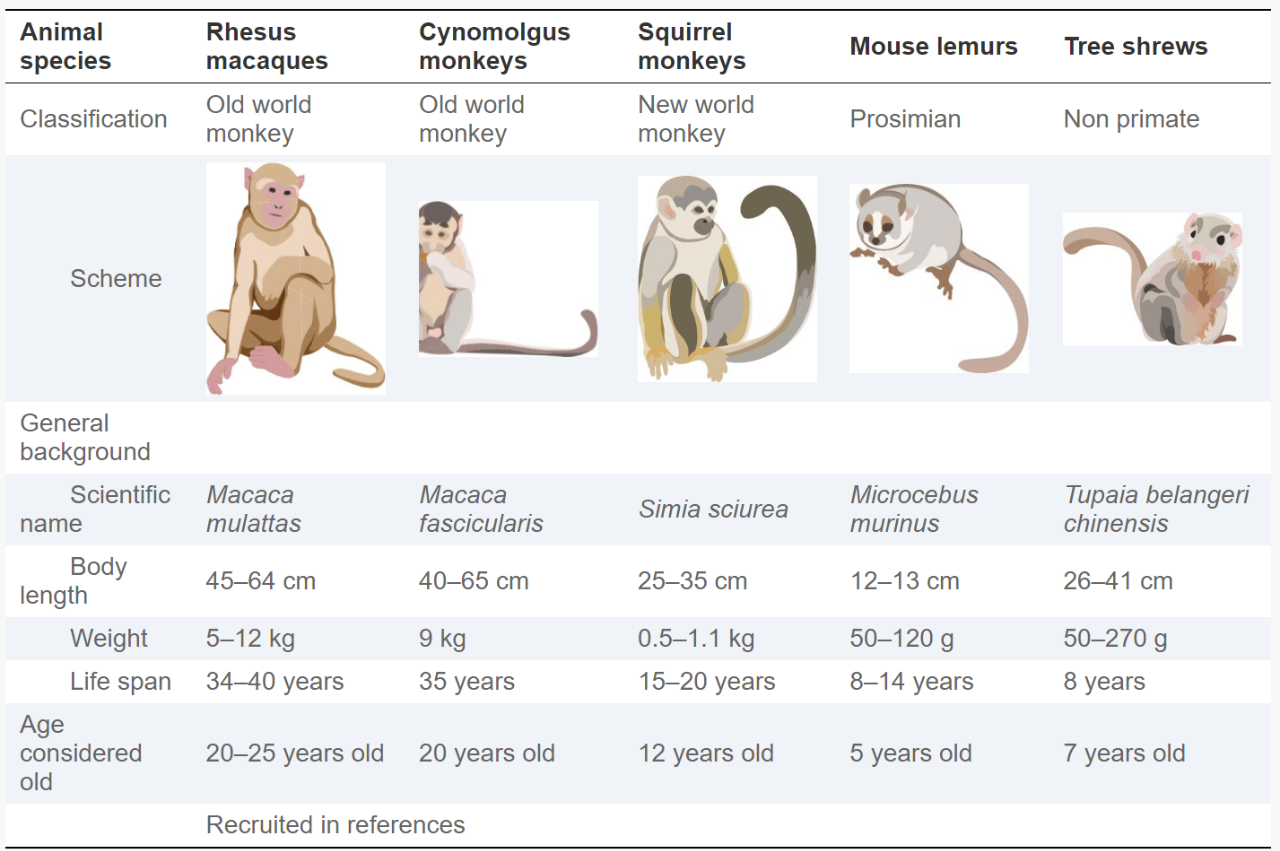
Table 1 The most widely used non-human primate (NHP) animal model in AD research.
Comprehensive Support from Creative Biolabs
Creative Biolabs offers a comprehensive NHP (Non-human Primate) biologicals, including Primary cells, biofluids, Tissues, nucleic acid, antibodies and custom services. Here is a simple list of our products and service. If you need more detailed information, please visit our home page.
- Non-Human Primate (NHP) Primary Cell
- Immortalized Non-Human Primate (NHP) Cell
- NHP Peripheral Blood Mononuclear Cell (PBMC)
- Blood-derived Non-Human Primate Biofluid Products
- Non-Human Primate (NHP) RNA Products
- Non-Human Primate (NHP) Cytosol Products
- Non-Human Primate (NHP) Tissue Block Products
- NHP Based Biomarker Discovery and Validation Service
- NHP Biospecimen Custom Collection Service
Advantages and Disadvantages of the NHP AD Model of Spontaneous (Natural Aging)
Human AD-like conditions found in older NHPs include rhesus monkeys, canine monkeys, squirrel monkeys, and baby lemurs (Table 2). While differences do exist between species, NHPs from these species exhibit extensive Aβ deposition in the parenchyma and cerebral vessels, some degree of Tau and p-Tau deposition in the parenchyma, rare intraneuronal accumulation of NFTs, and glial activation, restricted cerebral atrophy, and mild cognitive impairment in lemurs. Compared with other animal models of AD, naturally aging NHPs show high similarity to human AD due to their close phylogenetic relationship, similar neuroanatomy, comparable genetic profiles, and higher-order cognition Greater complexity of functionality. Therefore, naturally aging NHPs can be considered as a promising AD-like animal model that will improve our understanding of age-related cognitive impairment, pathogenic mechanisms, molecular and cellular interactions, and gene-environment interactions, further facilitating diagnosis Development of technological and therapeutic innovations.
However, the spontaneous NHP AD model also suffers from several major disadvantages. First, although naturally aged NHPs spontaneously exhibit Aβ proteopathies, they rarely exhibit AD-like NFTs or brain atrophy, suggesting the utility of using aged NHPs to analyze the interplay between Aβ, Tau, and neuronal death. Some difficulties. Second, cognitive deficits and executive dysfunction in elderly NHPs are much milder than in human AD, which may be an obstacle to evaluating the performance of novel treatments. Studying their protective mechanisms against severe NFTs, brain atrophy and cognitive decline has given us some inspiration. At the same time, they show homology to severe Aβ proteinopathy and apolipoprotein ε4. Finally, NHPs generally have a longer life expectancy compared with rodent models of AD, suggesting that longer observation periods are required before AD-like symptoms develop, adding to already high research costs. These shortcomings limit the widespread use of naturally aging NHPs as spontaneous AD models.
Advantages and Disadvantages of Induced NHP AD Models
Currently, ICV-Aβo, IT-Aβo, AD brain stock solution, ICV-STZ, methanol, and ICV-FA are commonly used to induce the AD model of NHP (Table 2). Elderly NHPs share significant similarities with humans, including neuroanatomy, neurophysiology, complex behaviors, and complex emotions, making them ideal models of human disease. The application of Aβo, AD brain stock solution, STZ and FA induces pathological changes such as Tau hyperphosphorylation (p-Tau), neuronal damage, brain atrophy, cognitive decline and memory impairment, which further fills the gap between natural aging NHPs and gap between human AD. Most importantly, injecting or feeding AD-related inducers can cause AD-related pathologies and behaviors to emerge in NHPs’ young adulthood rather than in the older stages of naturally aging NHPs, significantly reducing financial requirements and shortening experimental procedures.
However, all current induced AD modeling methods have their limitations. First, the doses, injection periods, and vaccination periods varied between studies, and the procedures were not standardized. Two well-designed studies of Aβo-induced NHPs that increased the dose, extended the injection period, and extended the vaccination period to two years may have exposed NHPs to all AD-like pathologies and symptoms, most importantly brain atrophy and cognitive decline. . The occurrence of brain atrophy and cognitive decline is barely observed in spontaneous NHP AD models, but they are severely affected in AD patients, indicating their importance in the development and validation of treatments for AD models. Secondly, the research design for constructing induced NHP AD models needs to be greatly improved. Many studies include only two or three NHPs in the treatment group, so AD-like pathology may be the result of chance. We encourage a more comprehensive assessment of induced NHPs, including examination of Aβ plaques, CAA, p-Tau, NFTs, glial activation, inflammatory conditions, neuronal death, brain atrophy, and behavioral changes. A more standardized evaluation of NHP models will facilitate better communication between studies, allow for the most efficient use of each study’s time and funding, and lead to a more efficient construction of a recognized, standardized NHP AD model. In addition, given the incomplete understanding of the etiology of AD, all artificially induced models are based on specific hypotheses of AD, making it difficult for an NHP model to fully reproduce the pathology and symptoms of AD. Building such a model should be one of the key points and difficulties in this field. Additional efforts are expected to improve methods, increase consistency, and ultimately standardize protocols for induced NHP AD models.
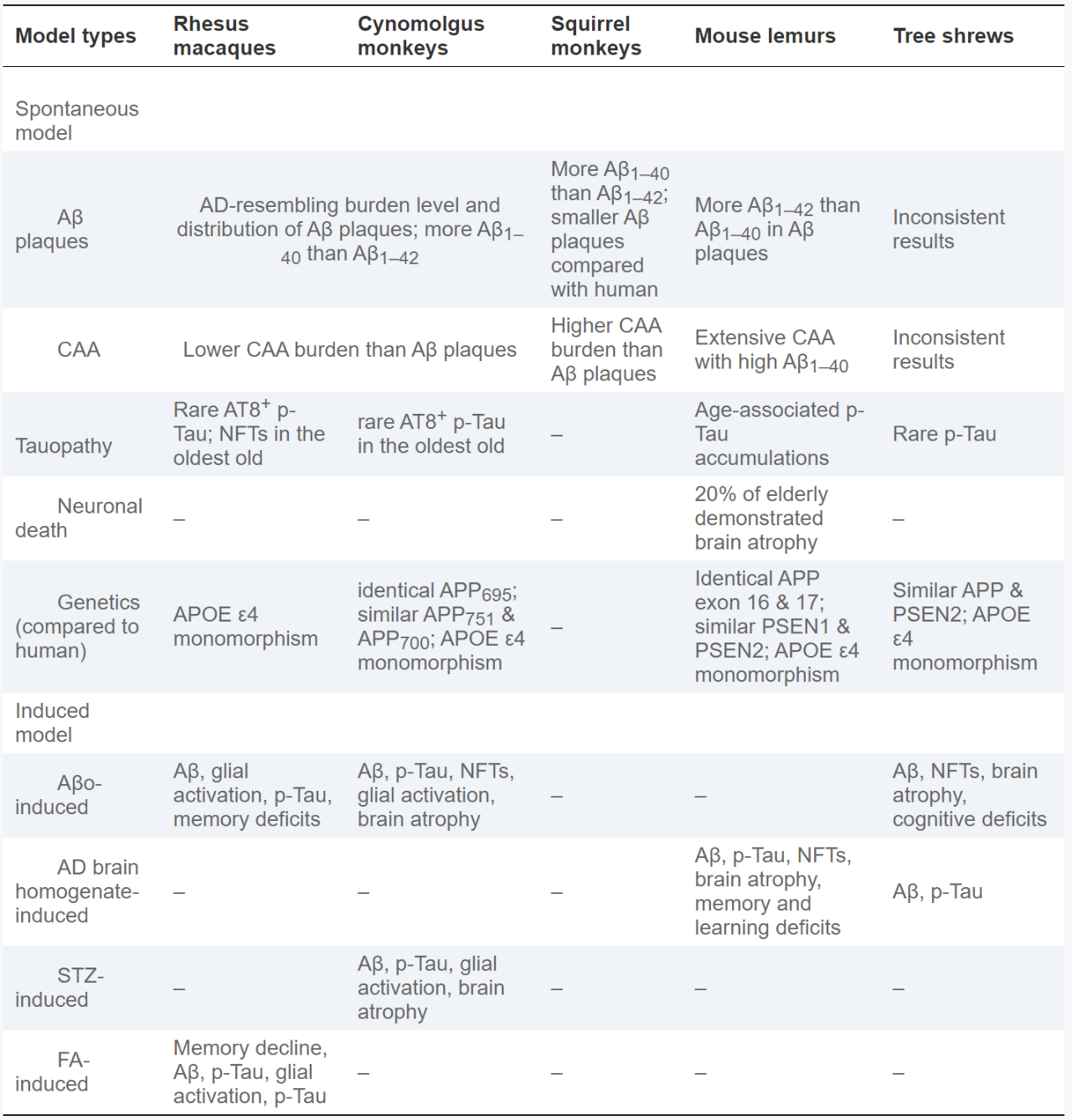
Table 2 Spontaneous (natural aging) and induced NHP AD models.
Conclusion
The review concludes with a look at the future direction of NPH models, anticipating that NHP models for AD will be used in a growing number of AD drug development programs to evaluate drug delivery pathways, nhp pharmacokinetics, pharmacodynamics, tolerability, safety, and most importantly, drug efficacy prior to Phase I clinical trials. Therefore, the current NHP AD model needs to be greatly improved, especially for drug efficacy evaluation in terms of imaging, laboratory biomarker, and behavioral testing. In the next few years, Aβ PET scans using appropriate radioisotope-labeled tracers, as well as new blood and urine biomarkers, will be applied. As such, these studies will provide the necessary data and solid evidence to facilitate clinical trials in humans.
Reference: Yihan Li., et al. ” Non-human Primate Models of Alzheimer’s Disease.” Journal of Exploratory Research in Pharmacology. 2023;8(3):199-221.
