Oncolytic viruses (OVs) have emerged as a very attractive cancer treatment modality. OVs target cancer cells without affecting normal cells, causing cancer cell lysis by replicating within cancer cells and, at the same time, activating the tumor microenvironment (TME).
Combining OV therapy with cancer immunotherapy has the potential to further reshape the TME and immune cell activation, enhancing the efficacy of immune checkpoint inhibitors against low- or non-responsive tumors.
Until now, OV therapy has been limited to injectable delivery to the tumor site, requiring a systemic anti-tumor response to be effective at non-injected sites. Intravenous administration of OVs can target all tumor sites, thereby improving efficacy. However, intravenous administration leads to the production of antiviral neutralizing antibodies in the human body, which severely limits the subsequent repeat dosing.
To maximize the potential of viral immunotherapy, strategies must be developed that avoid neutralization by antibodies in the human body. Retargeting, cellular vectors, polymeric coatings, and liposomes have been used to protect OVs from neutralizing antibodies, but these studies have not yet progressed to the clinical stage. In recent years, the widespread use of mRNA vaccines has paved the way for the development of lipid nanoparticle (LNP) carrier systems and holds promise for overcoming the challenges of intravenous drug delivery.
On October 7, 2022, researchers at Oncorus, an OV therapy development company, published a research paper in Nature Communications titled “Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer”.
The team developed an RNA virus by fully artificial synthesis, named Synthetic RNA virus, to deliver the RNA virus genome (vRNA) via LNP, which will address the limitations of repeated intravenous administration of OVs and increase the therapeutic potential of OV therapy.
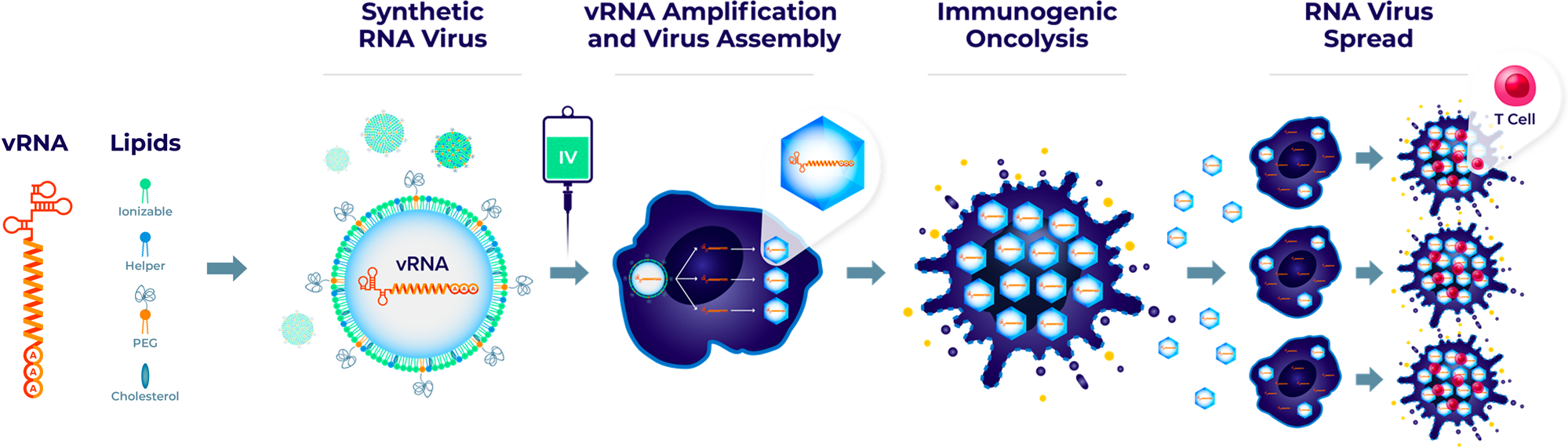
This synthetic virus is well tolerated and has demonstrated the ability to selectively kill tumor cells after intravenous administration in multiple tumor models, leading to tumor lysis and anti-tumor efficacy.
For this study, the team selected two viruses—Seneca Valley virus (SVV) and Coxsackievirus A21 (CVA21), which have good tumorolytic activity and clinical safety. The genomes of these two viruses are righteous single-stranded RNAs, which are sufficient to initiate the viral life cycle upon entry into tumor cells. This study reports the vRNA delivery and replication of synthetic SVV and CVA21.
The results show that this synthetic virus is well tolerated and has demonstrated the ability to selectively kill tumor cells after intravenous injection in multiple tumor models, leading to tumor lysis and anti-tumor efficacy, as well as enhancing the anti-tumor household type of PD-1 inhibitors. This synthetic viral platform will address the limitations of repeated intravenous administration of OVs and improve the therapeutic potential of OV therapy.
Reference
1. Kennedy, Edward M., et al. “Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer.” Nature Communications 13.1 (2022): 5907.
