Although checkpoint blockade therapy has achieved widespread success, it relies on reactive immune infiltration. Therefore, researchers are urgently seeking to develop therapies that enhance immune infiltration and prevent immune suppression. In a recent research report titled “An oncolytic virus-delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment,” published in the international journal Journal of Experimental Medicine, scientists from institutions including the University of Pittsburgh have discovered that infecting cancer with oncolytic viruses equipped with tumor-suppressive genetic vectors can stimulate the body’s immune system and help immunotherapy shrink or completely eliminate invasive tumors in mice. These findings may pave the way for clinical trials combining oncolytic viruses with immunotherapy.
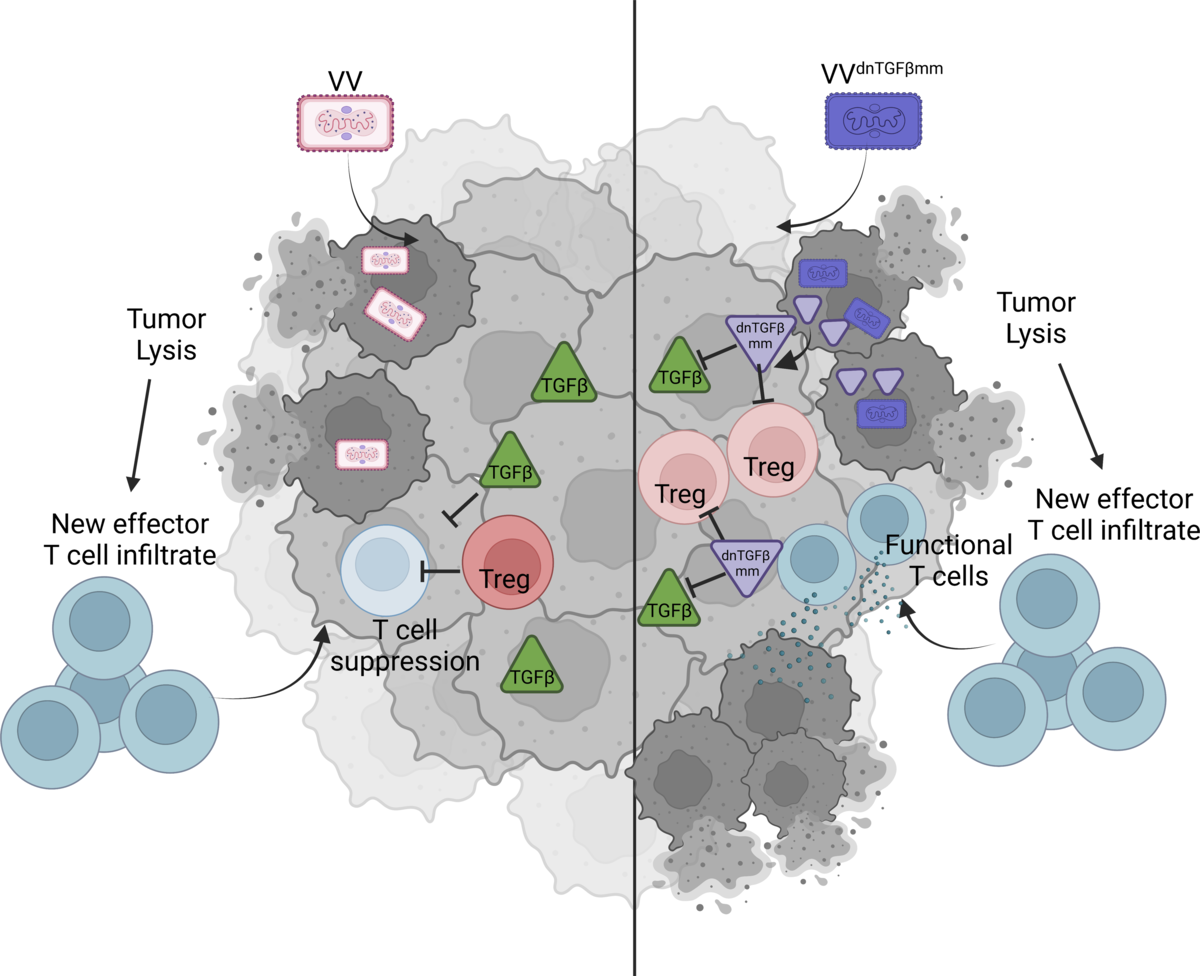
Oncolytic viruses are genetically modified viruses that can target rapidly dividing tumor cells while sparing normal cells. Originally designed to directly kill cancer cells, researchers later noticed that they can also stimulate the body’s immune system. This suggests that oncolytic viruses can be used in combination with other cancer therapies, such as immune checkpoint inhibitors. Immune checkpoint inhibitors can remove the “brakes” on the immune system so that T cells can recognize and attack tumors. Dr. Greg Delgoffe noted that immune checkpoint inhibitors only work in “hot” tumors, those that are already infiltrated by T cells. Oncolytic viruses can help “warm up” “cold” tumors, potentially synergizing with immunotherapy, although this goal has not yet been achieved by researchers.
The current challenge is that many patients’ tumors do not respond to oncolytic viruses. Researchers are very interested in laboratory-based research on oncolytic viruses, but it has not yet translated to clinical use. They also aim to understand the mechanisms behind tumors’ resistance to these viruses in order to determine how to help patients. In the study, researchers first developed head and neck squamous cell carcinoma (HNSCC) cell lines sensitive to oncolytic viruses, specifically vaccinia. When they injected the virus into these cells, the tumors regressed. They also developed a second cancer cell line that was otherwise identical but resistant to vaccinia. When they injected these two cell lines into mice and compared tumor growth and immunological differences, they found that vaccinia resistance might be driven by high levels of the signaling protein TGF-β, which is believed to promote cancer growth by suppressing the body’s immune environment.
In the next step, researchers modified vaccinia to carry a gene encoding a TGF-β inhibitor. Dr. Delgoffe stated that TGF-β inhibitors are very effective and have already been tested in clinical trials, but they often have some toxic effects because they are systemically delivered. What makes oncolytic viruses so promising is that they can deliver the “cargo” directly to the tumor microenvironment, making them highly targeted and a safer treatment approach. When researchers injected the modified vaccinia into mice with vaccinia-resistant HNSCC, the tumors shrank, and about 50% of the tumors in the mice completely disappeared. Importantly, this treatment did not cause any autoimmune reactions or toxicity-related side effects.
Next, researchers tested whether the modified virus would have a similar effect against highly invasive melanoma resistant to PD-1 immune checkpoint inhibitors. The results showed that untreated animals, those treated with anti-PD-1 therapy, or those receiving the control vaccinia all died in about 24 days. However, when the modified virus was administered, approximately 20% of the mice had completely cleared tumors. The most significant effect was observed when the modified virus was used in combination with anti-PD-1 therapy, with 67% of the mice completely clearing tumors, significantly extending their survival. Dr. Delgoffe and his colleagues hope that the modified vaccine virus, which has been licensed to the Kalivir Immunotherapy Company, will soon be used in human clinical trials as an adjunct to immune checkpoint inhibitors for patients who do not respond to immunotherapy.
In summary, the results of this study indicate that in addition to stimulating immune infiltration, oncolytic viruses also serve as a highly attractive method for delivering specialized agents and limiting immune suppression in cancer.
Reference
1. DePeaux, Kristin, et al. “An oncolytic virus–delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment.” Journal of Experimental Medicine 220.10 (2023): e20230053.
