Glioblastomas are aggressive brain tumors that are largely resistant to immunotherapy, perhaps related to immunosuppression and dysfunctional tumor vasculature, which tend to impede T-cell infiltration. LIGHT, which is also known as tumor necrosis factor superfamily member 14 (TNFSF14), induces high endothelial microvessels (HEVs) and tertiary lymphoid structures (TLS), which suggests that their therapeutic expression may promote T cell recruitment. In a recent study published in Cancer Cell entitled “Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma”, scientists from Uppsala University and other institutions have developed a new method that may help immune cells enter tumors from blood vessels to kill cancer cells, with the goal of improving the treatment of malignant brain tumors in humans.
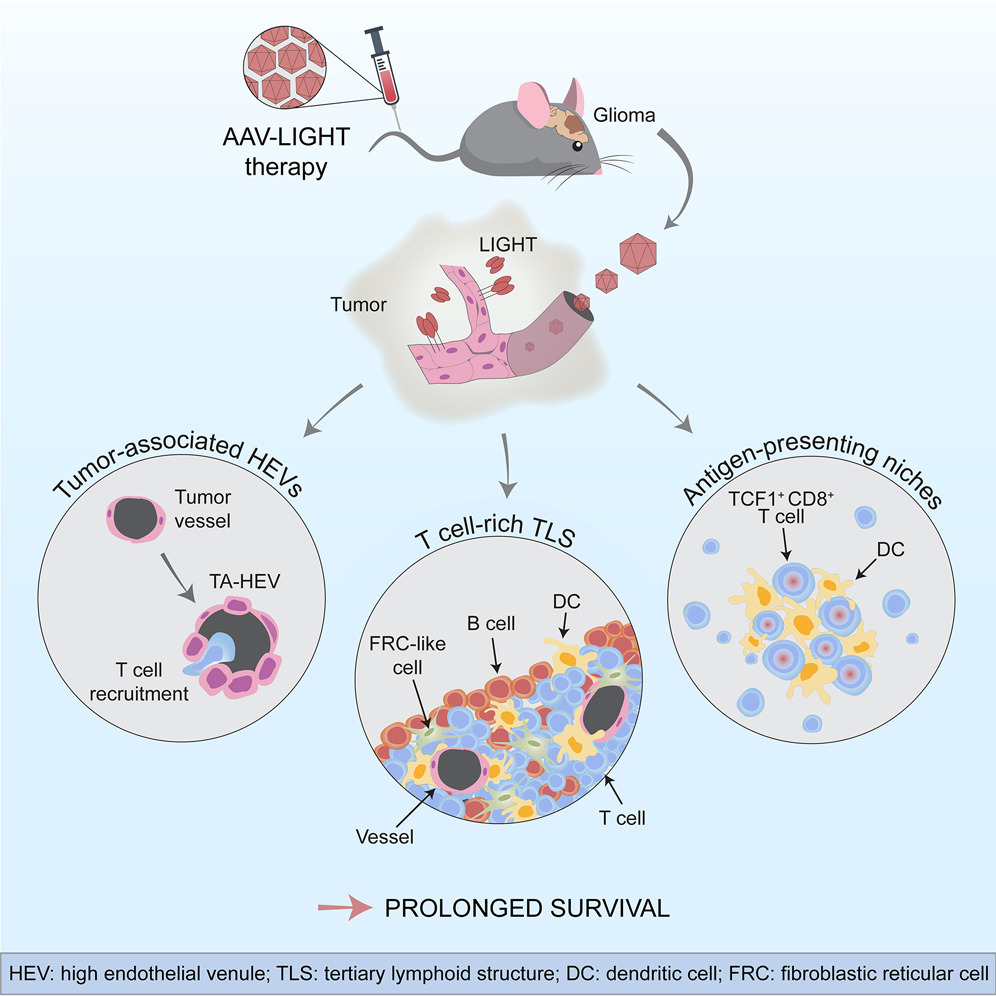
Glioblastoma is a malignant brain tumor that lacks effective therapies, in part due to the tumor’s ability to suppress or evade the body’s natural anti-cancer immune response. Immunotherapy can use checkpoint inhibitors to reactivate the body’s cancer-fighting immune system. However, in order for such therapies to be effective, special immune cells called killer T cells must be present inside the tumor. Unfortunately, blood vessels in brain cancer can become dysfunctional and act as a barrier to the entrance of killer T cells in the tumor, making immunotherapy ineffective against brain cancer.
In this latest study, the researchers devised a new method to help killer T cells access tumors and fight off cancer cells. They employed an adenovirus-associated virus (AAV) vector to specifically infect blood vessels in the brain and induce them to produce a factor called LIGHT, which alters the function of tumor blood vessels and increases their ability to transfer T cells from the blood to tumor tissue. “We found that when we used the viral vector AAV-LIGHT as a therapy in an experimental glioblastoma model, the tumor blood vessels changed their shape and function, and this viral vector induced the production of LIGHT in the blood vessels, thus using these functions to recruit killer T cells into the tumor, possibly creating a beneficial environment around the vessels that would support the function of killer T cells”, said researcher Anna Dimberg.
AAV-LIGHT also causes the creation of aggregates of immune cells associated with brain cancer, which resemble lymph nodes and whose presence in the tumor is associated with increased susceptibility to cancer immunotherapy. “We were very excited that TLS formed when we used AAV-LIGHT therapy, because the researchers believe that killer T cells can be activated to specifically target tumor cells in these structures”, said researcher Mohanraj Ramachandran, who also found that the therapy prolonged the survival of the experimental model and was even curative in some cases. In addition, AAV-LIGHT therapy distorts the composition of the TLS to induce the production of significant numbers of T cells.
According to the researchers, “we can manipulate the composition of the TLS with this novel therapy, so it may be promising as a tool to alter the function of these structures in a variety of situations. In addition, this novel therapy promotes the function of specific types of killer T cells, namely stem cell-like T cells found inside the TLS and the specific environment that are formed around tumor vessels. AAV-LIGHT therapy boosted the presence of stem cell-like T cells, which are expected to enhance immunotherapy efficacy, and looking for such cells in the TLS and special habitats may teach researchers about the importance of the interplay between AAV-LIGHT’s multiple impacts.” The researchers also intended to further investigate the development of novel therapies to establish whether AAV-LIGHT can be used to treat glioblastoma.
The researchers will need to further develop the viral vector before they beginning clinical studies, but the current findings are important, and they believe that this innovative therapy will help improve future treatment options for glioblastoma patients. To date, the biggest challenging of malignancies remains to be glioblastoma , because of its unique features, the Blood–Brain Barrier (BBB) and a locally immunosuppressive environment. Considering these limitations, oncolytic viruses allow to infect and kill glioma, with evidence that they may improve the efficacy of current standard therapy.
Reference
1. Ramachandran, Mohanraj, et al. “Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma.” Cancer Cell (2023).
