Cancer Immunotherapy & Oncolytic Virotherapy Combination Therapy Development Services
Introduction
Oncolytic viruses (OVs) are a promising class of cancer therapeutics that selectively replicate in and kill tumor cells. Several clinical studies have shown that single oncolytic virus therapy may not achieve satisfactory results, so oncolytic virus therapy is still combined with traditional treatment methods to achieve synergistic efficacy.
Oncolytic Virotherapy Development for Combination Therapy with Cancer Immunotherapy
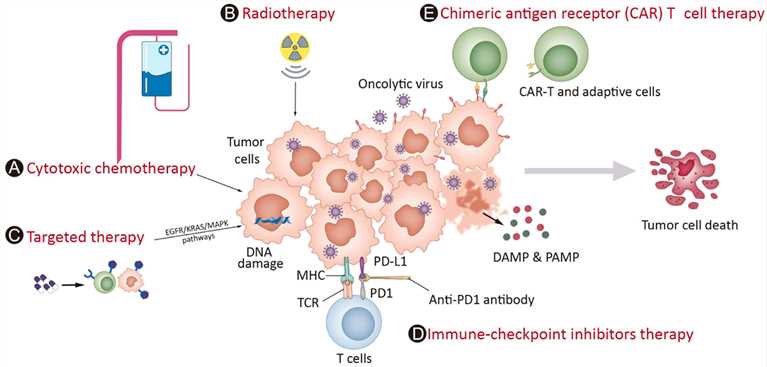 Fig.1 Strategies that can be used in combination with oncolytic viruses in the clinic.1,4
Fig.1 Strategies that can be used in combination with oncolytic viruses in the clinic.1,4
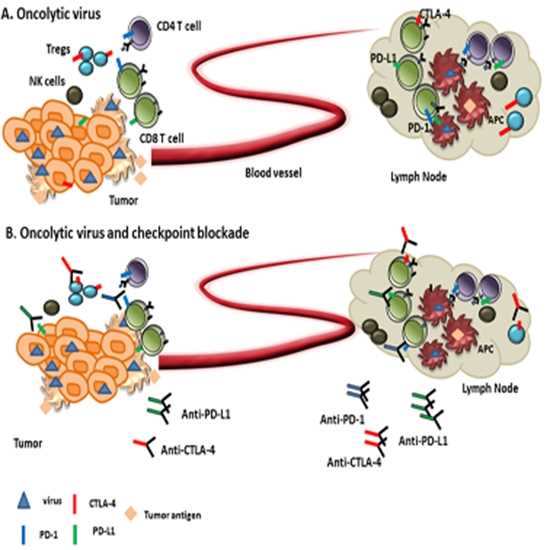 Fig.2 Oncolytic viruses in combination with immune
Fig.2 Oncolytic viruses in combination with immune
checkpoint inhibitors.2,4
Oncolytic viruses combined with immune checkpoint inhibitors can improve their anti-cancer effect. OV infection can cause tumor cells to release tumor-associated antigens and improve tumor immunogenicity. The genetically engineered OV can express immune stimulating factors in the tumor and enhance anti-tumor immunity. ICIs can inhibit the over-expressed immunosuppressive signal receptors of cancer cells and break the immunosuppression of the tumor microenvironment (TME). The combination of these two can promote a more potent immune reaction and show a stronger anti-tumor effect.
PD-1 and PD-L1 are effective in the treatment of some solid tumors, but the effect on complex tumors with immunosuppression is poor. The combination of ICIs and oncolytic viruses can improve the tumor's immune microenvironment. Both VG161 combined with PD-1 monoclonal antibody and CF33 combined with PD-L1 monoclonal antibody have shown good effects in preclinical studies based on animal models.
Radiotherapy (RT), a common cancer treatment, works by causing DNA damage and prompting cell apoptosis. When paired with oncolytic vaccinia virus (VACV), RT can set off necroptosis in tumor cells. By releasing damage-associated molecular patterns (DAMPs), this combination tweaks macrophages, creating a strong anti-tumor immune response and ramping up the overall anti-tumor effectiveness. Moreover, RT aids in the widespread distribution of oncolytic vaccinia virus throughout the body. Likewise, VACV carrying the gene for sodium-iodide symporter (NIS) can ferry radioiodine into cancer cells infected by the virus, further beefing up RT's tumor-killing power.
Chemotherapeutic drugs are toxic substances, that mainly operate by blocking DNA replication or messing with microtubule arrangements. When the oncolytic virus is combined with cisplatin or gemcitabine, its anti-tumor effectiveness gets stronger through various mechanisms, involving triggering apoptosis, changing the nucleotide levels, and interfering with DNA repair processes.
- Oncolytic virus combined with targeted therapy
Molecular targeted therapy takes specific molecular targets of tumor cells as the object of action. Target detection is used to clarify the patient's target situation, and targeted drugs (such as small molecule tyrosine kinase inhibitors, monoclonal antibodies, etc.) are used to develop personalized treatment plans, and the efficacy and adverse reactions are monitored during the treatment.
OVs can be used in combination with targeted therapies to attack cancer cells through multiple pathways, thereby potentially producing more durable responses. OVs can be used to deliver therapeutic genes that inhibit the same signaling pathways targeted by small molecule inhibitors, as well as to enhance the expression of target molecules to make tumor cells more sensitive to targeted therapies.
Cancer vaccines trigger an immune reaction against tumor cells by zeroing in on one or multiple antigens related to malignancies. These vaccines are designed to either cure existing cancers or inhibit cancer development. Oncolytic viruses can boost the effectiveness of cancer vaccines. Moreover, they can leverage established tumors as a local supply of neoantigens for vaccination via cross-presentation, leading to the shrinkage of distant tumors that have not been directly infected.
- Oncolytic virus in combination with chimeric antigen receptor T cells or NK cells
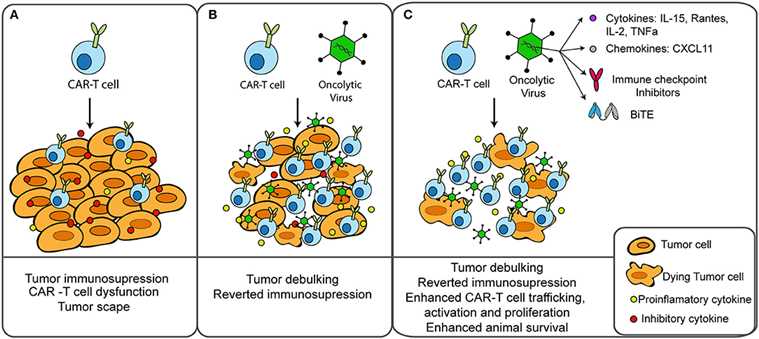 Fig.3 Oncolytic virus combined with CAR-T therapy for tumor treatment.3,4
Fig.3 Oncolytic virus combined with CAR-T therapy for tumor treatment.3,4
OVs can be combined with CAR-T or NK cell therapies to improve tumor cell recognition and killing, particularly in solid tumors. CAR-T cells are engineered to express a receptor that specifically recognizes a tumor-associated antigen, allowing them to target and kill cancer cells. However, in some solid tumors, the expression of these target antigens can be heterogeneous, limiting the efficacy of CAR-T cell therapy. OVs can enhance the expression of these antigens or spread throughout the tumor, ensuring that more cancer cells are targeted by the CAR-T cells. Similarly, OVs can enhance the activity of natural killer (NK) cells, another type of immune cell that can kill cancer cells. OVs can stimulate the release of cytokines that activate NK cells and make them more effective at recognizing and destroying tumor cells.
-
Oncolytic virus in combination with adoptive T cells
Adoptive T cell therapy (ACT) entails administering to patients a substantial quantity of T cells with either complete or partial tumor-reactive specificities. OVs can aid in ACT by priming both the systemic immune system and the local TME, thereby creating a more favorable condition for T cell infiltration and the execution of their effector functions. Studies have demonstrated that the combination treatment outperforms either treatment alone by a significant margin, primarily due to the enhanced effector capabilities of T cells.
The following are several therapies:- OVs for T Cell Retargeting
- OVs Expressing Cytokine/Chemokine
- OVs combine with Tumor-Infiltrating lymphocyte Therapy
- OVs combine with T-cell Receptor-engineered T-cells Therapy (TCR-T)
Creative Biolabs provides comprehensive services for developing oncolytic virotherapy-based combination therapies, offering expertise in virus design, preclinical testing, and data analysis to accelerate your cancer research.
References
- Chen, Lingjuan, et al. "Oncolytic virotherapy in cancer treatment: challenges and optimization prospects." Frontiers in Immunology 14 (2023): 1308890. DOI: 10.3389/fimmu.2023.1308890.
- Rajani, Karishma R., and Richard G. Vile. "Harnessing the power of onco-immunotherapy with checkpoint inhibitors." Viruses 7.11 (2015): 5889-5901. DOI: 10.3390/v7112914.
- Guedan, Sonia, and Ramon Alemany. "CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge." Frontiers in immunology 9 (2018): 2460. DOI: 10.3389/fimmu.2018.02460.
- Distributed under Open Access license CC BY 4.0, without modification.
