Targeted protein degradation (TPD) has emerged over the past decade as a major novel drug modality for the removal of intracellular proteins with bispecific small molecules that recruit proteins of interest (POIs) to E3 ligases for degradation in the proteasome. Unlike traditional mass-occupancy-based drugs, intracellular TPD (iTPD) eliminates the target and catalyzes action, so it can be more effective and sustained with lower dosage requirements.
Recently, this approach has been extended to the extracellular proteome, including secreted proteins and membrane proteins. Extracellular protein-targeted degradation (eTPD) utilizes bispecific antibodies, conjugates, or small molecules to transport extracellular POIs to lysosomes for degradation. Here, we highlight the latest advances in eTPDs, including degradation systems, targets, molecular design, and parameters to advance them. Almost any protein, intracellular or extracellular, can now be treated with TPD in principle.
1. Intracellular protein-targeted degradation (iTPD): PROTACs and molecular glues
In recent years, intracellular targeted protein degradation (iTPD) has emerged as an important new model for small molecule drugs (Figure 1). At present, two classes of such molecules have been developed. The first type is proteolysis-targeting chimeras (PROTACs), which are large bispecific small molecules conjugated by a linker. They induce POI ubiquitination and the tagged disease causing protein will be ultimately degraded by the proteasome in the cytoplasm. The second type is known as molecular glue, which enhances the interaction between E3 ligase and POI, leading to its ubiquitination and proteasomal degradation. iTPD offers significant advantages over traditional “mass drive” based inhibitors. First, PROTACs, or molecular glues, can theoretically bind anywhere on a POI, whereas inhibitors often require binding to active or allosteric sites. Second, the degradation of the target removes the entire protein, including its scaffold function, so the therapeutic activity of the drug should last longer and more closely follow the results of knockout experiments. Third, these molecules are catalytic and not stoichiometric, as in classical pharmacological inhibitors, so the same therapeutic effect can be achieved at low doses. There are currently more than two dozen clinical trials involving PROTACs and molecular glues, some of which have entered Phase II and Phase III studies.
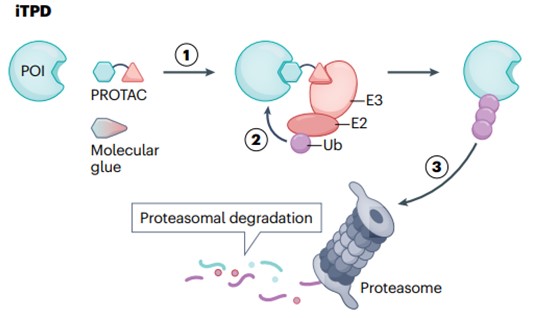
Fig. 1 General mechanisms of intracellular targeted protein degradation pathways1
2. Extracellular Protein Targeted Degradation (eTPD): An Emerging Drug Modality
The iTPD of the cytosolic protein has stimulated research into the extracellular proteome in biology and chemistry, known as extracellular TPD (eTPD). In addition to the location of the target POI, there are at least three significant differences between iTPD and eTPD (Figure 2).
First, cells recycle proteins through two main pathways: the proteasomal and lysosomal pathways. iTPD is almost entirely dependent on the proteasome pathway, which is commonly used to process intracellular proteins. eTPD involves bispecific biologics or small molecules that recruit membrane-bound or secreted POIs to membrane-bound circulating receptors and deliver POIs to lysosomes, which are typical pathways for extracellular protein degradation.
Second, these two proteolytic disruption modes have different kinetics and use different proteases. iTPD is faster—usually within a few minutes to a few hours because all the components are inside the cell. In contrast, the rate of eTPD is generally slower, typically 6–48 hours, as it involves vesicle transport from the membrane through early and late endosomes, and eventually fusion with lysosomes, leading to protein degradation.
Third, almost all iTPD systems use only a few E3 ligases, mainly CRBN or VHL, which present challenges in finding E3 ligase conjugates. Both ligases are widely expressed in tissues, which limits tissue-selective targeting. In contrast, eTPD can use a variety of degradation systems, which allows for more specific tissue selectivity.
Finally, the pharmacokinetics of antibodies are very long compared to typical small molecules, so they are administered less frequently.
Therefore, eTPD represents an emerging new paradigm for biologics development.
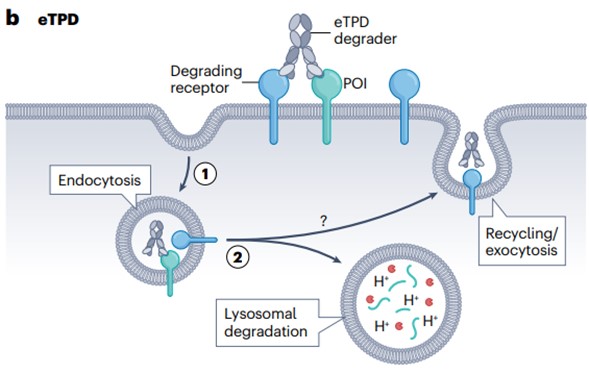
Fig. 2 General mechanisms of extracellular targeted protein degradation pathways1
3. Six different strategies for eTPD
At present, there are six different methods and strategies for eTPD, including eTPD—a clearance antibody that degrades soluble POI, eTPD—a circulating receptor eTPD based on glycans, eTPD based on transmembrane E3 ligase targeting membrane proteins, eTPD based on cytokines, eTPD based on integrin, and eTPD that degrades membrane proteins from the inside.
- Clearance antibody eTPD that degrades soluble POI
The Fc region of the antibody has an important function for antibody circulation and immune cell activation. Nascent Fc receptors (FcRns) are responsible for recovering antibodies internalized into endosomes, sending them back outside the cell before they reach lysosomal degradation, and enhanced binding of antibodies to FcRn can extend their half-life in human serum to 21 days.
The “scavenging antibody” is designed so that FcRn delivers POI to acidic endosomes in a pH-switchable manner, releasing POI for lysosomal degradation (Figure 3a). This is inspired by the natural circulating mechanism of low-density lipoprotein (LDL) uptake, which binds to the LDL receptor and is released into endosomes at low pH for lysosomal degradation. Tocilizumab (Tcz), a humanized antibody approved for the treatment of rheumatoid arthritis, binds pH-dependently to the IL-6 receptor (IL-6R). By redesigning Tcz as a clearing antibody, its affinity for FcRn was increased by utilizing a known mutation, and then by mutations in the complementary deterministic region CDRs, the affinity for IL-6 was reduced by approximately 20-fold at pH 6.0 while maintaining affinity at pH 7.4. The modified antibody can deliver IL-6R to the lysosome while maintaining attachment to FcRn and cycling back to the plasma membrane to collect more IL-6. pH-switchable Tocilizumab was approved in 2020 for the treatment of neuromyelitis optica spectrum disorder, representing the first approved antibody in eTPD.
- Glycan-based circulating receptor eTPD
The use of glycan-targeted recycling receptors, such as cationic independent mannose 6-phosphate receptor (CI-M6PR) or sialic acid glycoprotein receptor (ASGPR), can promote lysosomal degradation of membranes and soluble POIs (Figure 3b). One approach, known as lysosome-targeted chimerism (LYTAC), involves the bioconjugation of multiple glycan ligands of CI-M6PR to antibodies targeting POIs, and the LYTAC-POI complex binds to CI-M6PR and is internalized, resulting in the degradation of POIs in lysosomes. Another approach, known as Bifunctional Small Molecules of MoDE or ASGPR-Targeting Chimeras (ATACs), is being used to develop a similar ASGPR-based approach.
CI-M6PR is a 300 kDa dimer type I receptor, a pH switchable receptor that binds to ligands at neutral pH and is released in acidic endosomes before fusion with lysosomes. CI-M6PR has been used to shuttle M6P-carrying exogenous lysosomal enzymes to lysosomes for the treatment of lysosomal diseases. ASGPR is predominantly highly expressed on hepatocytes and rapidly clears non-sialylated glycoproteins. In conclusion, glycan-based eTPD degradation methods mainly utilize ASGPR or CI-M6PR.
- eTPD based on transmembrane E3 ligase targeting membrane proteins
In addition to the 600–700 intracellular members of E3 ligases, there is a family of E3 ligases containing transmembrane domains with about 30 members. Target proteins can be degraded using bispecific antibodies targeting this family member and POI, known as antibody-based protein degradation-targeting chimeras (AbTACs), protein degradation-targeting antibodies (PROTABs), or receptor elimination (REULR) recruited by E3 ubiquitin ligase (Figure 3c). In one study, an AbTAC of PD-L1 was constructed, with the POI arm selecting the Fab domain of atezolizumab bound to PD-L1 and the degradation arm using the Fab of the RNF43 extracellular domain. Atz-AbTAC adopted the classical KICH mode. Atz AbTAC induced the degradation of PD-L1 with a DC50 of 3.4 nM, a 24-hour maximum degradation (Dmax) of approximately 63%, and whole-cell proteomics showed no significant overall changes in the cellular proteome and no significant cytotoxicity.
In conclusion, it has been shown that bispecific molecules (AbTACs, PROTABs, and REULRs) with members of the transmembrane E3 ubiquitin ligase family can co-selectively degrade some important membrane proteins for therapeutic purposes.
- eTPDs based on cytokine-targeting membrane proteins
It is well known that many cytokines can be absorbed and degraded by their receptors by shuttling to lysosomes. This provides another opportunity to harness the endogenous mechanism of eTPD in a method called cytokine receptor-targeting chimera (KineTAC) (Figure 3d). KineTACs bind to natural cytokines or growth factors with one arm, while the other arm binds exclusively to POIs. This allows it to work against membrane-bound and soluble proteins for efficient and specific protein degradation.
Like AbTAC, KineTAC can degrade a wide range of membrane proteins in a wide range of cell types. And similar to LYTAC, they can also degrade soluble proteins because cycling is not dependent on ubiquitination. In addition, the transcription level of POI can be significantly higher than that of degradants and can still be efficiently degraded.
- Integrin-based eTPD
Inspired by the targeted delivery of anticancer drugs using integrin αVβ3, eTPD introduces an integrin-based degradation system (Figure 3e). It involves the use of bispecific antibodies that bind specifically to integrins with one arm and the other to POIs. Directs POIs to lysosomes for degradation while harnessing the cycling capacity of integrins to enhance target localization and efficiency.
As proof of principle, the biotin-binding protein NeutrAvidin was targeted using an integrin αVβ3-binding biotin chimera. This molecule contains a cyclic RGD motif (cRGD) covalently linked to biotin, which successfully internalizes NeutrAvidin in A549 cells within 20 h. Overall, this integrin-based degradation system shows exciting potential for tumor-selective degradation of membranes or soluble proteins.
- Degradation of membrane proteins from the inside of eTPD
Receptor tyrosine kinase (RTK) has been studied for PROTAC targeting, which contains a canonical E3 ligase conjugate of CRBN or VHL linked to various RTK inhibitors (Figure 3f). This PROTAC binds in part to the intracellular kinase domain of the receptor tyrosine kinase (RTK), while the other part binds to the E3 ligase. As a result, the receptor tyrosine kinase is ubiquitinated, and the receptor tyrosine kinase is degraded in the proteasome. In summary, the use of PROTACs conjugated to VHL or CRBN can degrade RTKs internally. Although this approach is still in its infancy, PROTACs using intracellular domains targeting membrane proteins have shown promising applications.
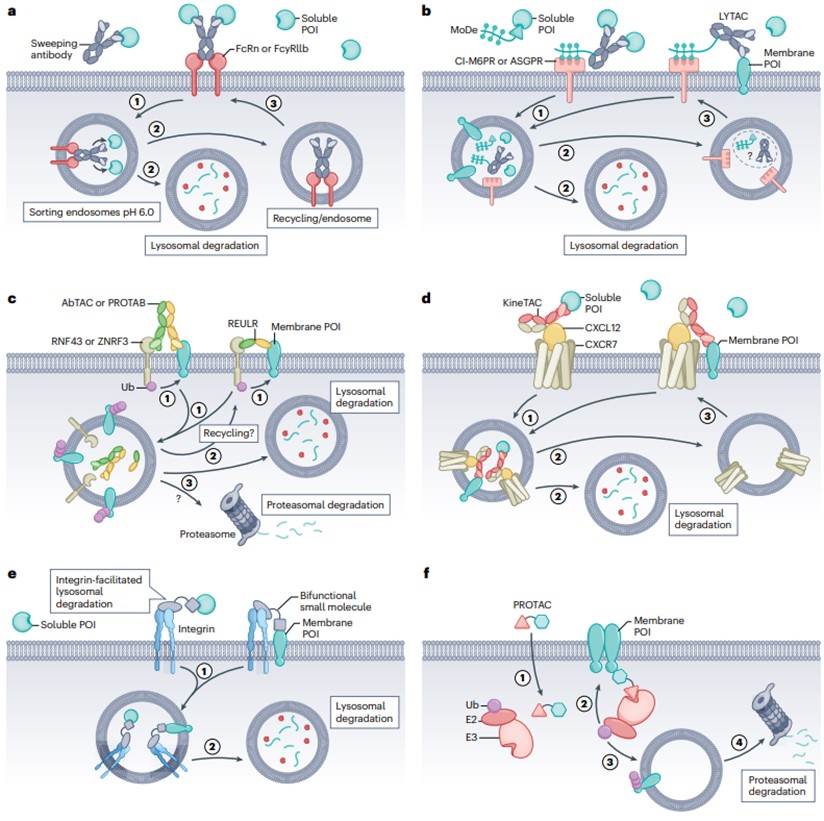
Fig. 3 Six different methods for degrading extracellular proteins1
The eTPD field is in its infancy and is rapidly following the development of the iTPD field, with various designs and methods currently being studied. However, there are still many important issues to be solved in the field of eTPD.
References:
- Wells, James A., and Kaan Kumru. “Extracellular targeted protein degradation: an emerging modality for drug discovery.” Nature Reviews Drug Discovery 23.2 (2024): 126-140.
