Targeted protein degradation (TPD) primarily degrades target proteins through the ubiquitin-proteasome and lysosome, and according to the specific mechanism of action, it can be further subdivided into nearly 10 different technical routes, among which the fastest developing are molecular glue and PROTAC technology.
The molecular glue of BMS has reached an annual sales volume of 12.891 billion US dollars, while Arvinas’ PROTAC molecule ARV-471 has already initiated phase III clinical trials. Targeted degradation based on lysosomes has a shorter development time and is still in the pre-clinical stage.
Under the current drug development situation where conventional target development is exhausted and it is difficult to find new targets, TPD technology provides a new pathway for drug development, greatly broadening the range of target proteins and is expected to be one of the most promising technologies for the future.
1. Differences between PROTAC and molecular glue
Proteolysis Targeting Chimeras (PROTACs) and molecular glues are two rapidly developing technologies for targeted protein degradation in recent years. They achieve protein degradation in disease treatment through different mechanisms, and both have the potential to change traditional drug development methods.
① Different Structures: PROTACs generally consist of three parts: a target protein ligand, a linker, and an E3 ubiquitin ligase ligand. This structural design enables PROTAC molecules to simultaneously connect with the target protein and E3. Molecular glues, on the other hand, are usually single, small molecules. They promote interactions between them by altering the conformation of the target protein or E3 ubiquitin ligase, without the need for specific linkers or high-affinity ligands for the target protein (Figure 1).
② Different Mechanisms: The main difference between PROTACs and molecular glues lies in their mechanisms of action. PROTACs induce target protein degradation by linking the target protein to the E3 ligase ligand, allowing the ubiquitin attached to the target protein to enter the proteasome for degradation. This method can thoroughly eliminate pathological proteins, thereby offsetting their disease activity. However, molecular glues form a stable complex by stably interacting with the pathological protein, thereby inhibiting its activity or degrading the target protein.
③ Different Efficiencies: PROTAC can achieve selective degradation of target proteins, and its efficacy depends on the affinity between PROTAC and the target protein and E3 ligase. Unlike traditional enzyme inhibitors or receptor blockers, PROTAC not only inhibits protein function but also reduces protein quantity. Molecular glues can also promote the degradation of target proteins. Since this is achieved by directly binding the target protein with the E3 ligase, its efficiency depends on the affinity between the molecular glue, the target protein, and the E3 ligase (Table 1).
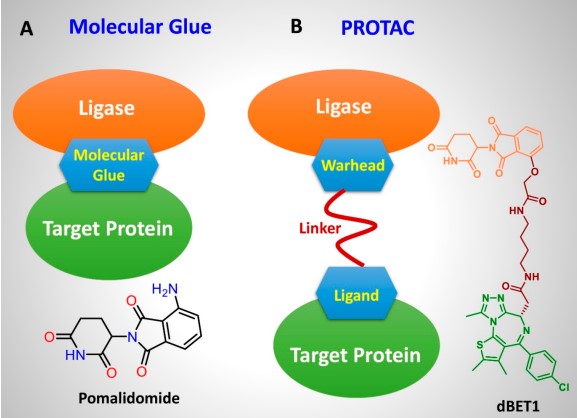
Fig.1 Mode of action and structural features of molecular glue and PROTAC
| Molecular glue | PROTAC | |
| Mechanism | Inducing PPI by binding to the E3 enzyme or target protein | Binding target and E3 enzyme |
| Target protein (POI) | Undetermined | Predictable |
| Discovery strategy | Discovered by chance in history | Reasonable design |
| Feature | Unit price | Divalent |
| Linker | No need to connect sub | Connection required |
| Molecular weight | Low | high |
| Lipinski’s Five Rules | Accord with | Not compliant |
| Target protein binding pocket | Non essential | Required |
| Affinity | The affinity for the E3 enzyme and POI is not very strong. | Strong affinity for the E3 enzyme and POI |
Table 1: The difference between molecular glue and PROTAC
2. The Cutting-Edge Advances of PROTACs and Molecular Glues
To date, PROTACs have been successfully used in the research of various types of protein-related diseases, showing strong therapeutic effects in clinical trials. For example, there’s ARV-471 which targets estrogen receptors, and MT-802 which targets BTK. However, there are currently no PROTAC drugs approved worldwide.
With Arvinas’ two candidate molecules, ARV-110 and ARV-471, taking the lead in obtaining positive clinical data, this field has experienced robust growth in recent years. Currently, several PROTAC drugs have entered the clinical stage, with targets including AR, ER, BCL-XL, IKZF1/3, STAT3, BTK, TRK, BRD9, etc.
| PROTAC | Target points | Indications | Research stage |
| ARV-471 | Estrogen receptor (ER) | Breast cancer (ER+/HER2 BreakCancer) | Phase III |
| ARV-110 | Androgen receptor (AR) | Metastatic castration resistant prostate cancer (mCRPC) | Phase II |
| ARV-766 | Androgen receptor (AR) | Metastatic castration resistant prostate cancer (mCRPC) | Phase II |
| KT-474 | Interleukin-1 receptor associated kinase 4 (IRAK4) | Allergic dermatitis, purulent sweat gland inflammation, rheumatoid arthritis | Phase II |
| LNK01001 | – | Rheumatoid arthritis, atopic dermatitis, ankylosing spondylitis | Phase II |
| GT20029 | AR | Androgenic alopecia | Phase II |
| CFT7455 | IKZF1/3 | Recurrent/refractory non Hodgkin’s lymphoma or multiple myeloma | PhaseI/I |
| CFT1946 | BRAFV600 | BRAFV600 mutated solid tumor | PhaseI/I |
| NX-2127 | BTK+IKZF | B-cell malignant tumor | Phase I |
| NX-5948 | BTK | B-cell malignant tumors and autoimmune diseases | Phase I |
| LNK01002 | – | Hematological tumors | Phase I |
| LNK01003 | – | Immunity and inflammation | Phase I |
| HSK29116 | BTK | B-cell malignant tumor | Phase I |
| MZ-001 | BTK | B-cell malignant tumors and autoimmune diseases | IND declaration |
| HP518 | AR | MCRPC with standard treatment failure | IND declaration |
| CFT8919 | EGFR L858R | Non small cell lung cancer (NSCLC) with drug-resistant EGFR mutations | Preclinical |
| CFT8634 | BRD9 | Synovial sarcoma and solid tumors with smarcb1 deficiency | Precision |
| KYM-001 | KYM-001 | MYD88 gene mutation in B-cell lymphoma | Precision |
| CG416 | Neurotrophic factor receptor tyrosine kinase (TRK) | – | Preclinical |
| CG428 | TRK | – | Precision |
| CG001419 | TRK | – | Precision |
| HC-X029 | AR sv | End line treatment for mCRPC with failed standard treatment | Precision |
Table 2: PROTACs currently in the clinical stage
In contrast, molecular glues as a relatively new therapy, are less researched and developed but have already demonstrated therapeutic potential in certain specific instances. The molecular glues that have been approved for clinical use are primarily immunomodulators, such as Thalidomide, Lenalidomide, and Pomalidomide, used to treat conditions such as multiple myeloma and myelodysplastic syndromes. The molecular weights of these three molecular glue degraders are all below 300 Da, and they all degrade target proteins, including the transcription factor IKZF1/3, by recruiting the E3 ubiquitin ligase CRBN. In addition, thalidomide analogs are often used as ligands for CRBN in many PROTAC molecules. For example, the E3 ligase ligand in the phase III clinical drug ARV-471 is (R)-thalidomide.
3. Future Development Trend of PROTAC and Molecular Glue
Although there are currently no PROTAC drugs on the market, several drugs that have preliminary human clinical data have been proven to significantly degrade intracellular proteins with good therapeutic effects. However, confirming efficacy is a slow process, and more extensive sample data needs to be gathered to verify its validity. Due to its unique mechanism of action, PROTAC technology is attracting more and more biopharmaceutical innovators and entrepreneurs to compete on this new track. Over the course of more than 20 years of development, PROTAC has broken through the widely accepted drug development rules, opening a new chapter for drug development in its continuous exploration and advancement. It is expected that PROTAC technology will have a wider application in future drug research and development. Meanwhile, with technological enhancements, the design and preparation process of PROTAC will become more precise and controllable.
Currently, more than 600 types of E3 ligases have been reported, but only five have been used for molecular glue-mediated degradation, namely CRBN, DDB1, β-TrCP, DCAF15, and SIAH1. The E3 ligase library still has immense potential waiting to be explored. Identifying new E3 ligase ligands helps expand our degradable target proteins. Moreover, the chemical space of molecular glue drug molecules also merits further exploration. The majority of molecular glues reported to date still share a high degree of similarity with thalidomide and its derivatives. Certainly, this poses a significant challenge for drug developers who need to deepen their understanding of protein-protein interaction interfaces and create more rational structure-guided molecular glue designs, pushing molecular glues into clinical use and aiding in the treatment of more diseases.
Reference:
Dong G, Ding Y, He S, et al. Molecular glues for targeted protein degradation: from serendipity to rational discovery[J]. Journal of medicinal chemistry, 2021, 64(15): 10606-10620.
