N 6-methyladenosine (m6A) methylation is the most abundant type of RNA modification, primarily catalyzed by the METTL3-METTL14 methyltransferase complex (MTC). This complex includes the core catalytic proteins METTL3 and METTL14, as well as several regulatory proteins, including WTAP, VIRMA, HAKAI, ZC3H13, and RBM15. METTL3 is the sole catalytic component, while METTL14 primarily maintains the integrity of the complex and promotes the binding of substrate RNA.
The methyltransferase complex is associated with various diseases, including cancer, cardiovascular disease, and neurological disorders. As the core catalytic subunit of MTC, METTL3 is overexpressed in various types of cancer, such as hematological malignancies, lung cancer, and liver cancer, and is considered a key oncogenic driver in malignant myeloid hematopoietic cells. Studies have revealed that METTL3 sustains the leukemic state of acute myeloid leukemia (AML) by catalyzing the methylation of mRNA encoding oncogenic transcription factors. Inducible loss of METTL3 has been shown to lead to cell cycle arrest and impaired differentiation and growth in AML cells. In addition, METTL14 is also overexpressed in AML cells and promotes leukemia occurrence by regulating MYB and MYC through m6A modification.
Given the pivotal role of the METTL3-METTL14 complex in tumorigenesis, the development of specific METTL3-METTL14 inhibitors is an emerging and promising research area. Up to now, only a few inhibitors targeting the catalytic activity of the METTL3-METTL14 complex, such as UZH2 and STM2457, have been developed, which are SAM-competitive inhibitors of METTL3. STM2457 has exhibited potent anti-leukemic potential in AML cell lines without noticeably affecting normal hematopoiesis. These findings suggest that the METTL3-METTL14 complex is an appealing target for cancer therapy, especially for AML. However, there is a lack of research on METTL3 inhibitors targeting its catalytic activity.
Recently, researchers from the Shanghai Institute of Materia Medica have developed a selective proteolysis-targeting chimera (PROTAC) for the METTL3-METTL14 complex, WD6305.
Other metabolic enzymes targeting PROTACs
Their study results show that WD6305 more effectively inhibits m6A modification and AML cell proliferation compared to its parent inhibitor, UZH2, and promotes cell apoptosis. Furthermore, they found that WD6305 affects several signaling pathways associated with the development and proliferation of AML. These findings indicate that targeted degradation of the METTL3-METTL14 complex is a promising AML treatment strategy (Figure 1).
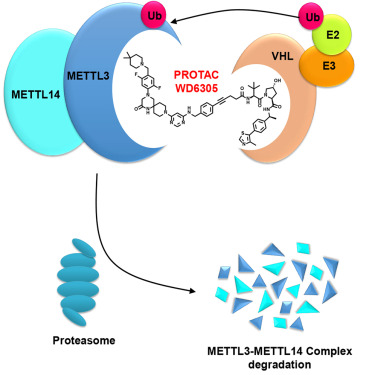
Figure 1. Schematic diagram of METTL3-METTL14 complex PROTAC action. (Du W, 2024)
In this study, the researchers attempted to develop an efficient therapeutic tool to disrupt the stability of METTL3 and/or METTL14. Using UZH2, a METTL3 inhibitor, as a binding ligand, they designed and synthesized a series of METL3-targeting PROTAC molecules. The researchers then utilized Western Bolt to investigate the degradation capabilities of these compounds in MonoMac-6 cells, an AML cell line expressing METTL3. The experimental results indicated that compound 9 (WD6305) had the strongest degradation ability (Figure 2). Following this, the researchers selected WD6305, the compound that exhibited the most efficient degradation, to further characterize its degradation efficiency on METTL3. Western blot and proteomics analyses revealed that WD6305 reduced the levels of the METTL3 and METTL14 proteins in a dose-dependent and selective manner in MonoMac-6 cells (Figure 3). The entirety of these research findings verified that WD6305 is an efficient, selective, fast-acting, and enduring degrader of METTL3 and effectively removes the METTL3-METTL14 complex.
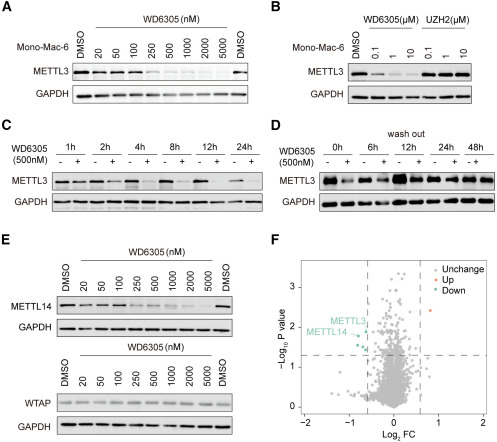
Figure 2. Degradation profiling of METTL3-based degrader (Du W, 2024)
Next, the researchers confirmed the mechanism by which WD6305 degrades the METTL3-METTL14 complex. The study demonstrated that WD6305 degrades the METTL3-METTL14 complex via the ubiquitin-proteasome system. Lastly, the researchers assessed the anti-leukemic potential of WD6305 in several AML cell lines, including Mono-Mac-6 and MOLM-16. The study results show that WD6305 displayed stronger anti-proliferative activity and could more effectively induce apoptosis in Mono-Mac-6 cells than UZH2 (Figure 3). These findings suggest that, compared to its parent METTL3 inhibitor, WD6305 has superior anti-leukemic activity.
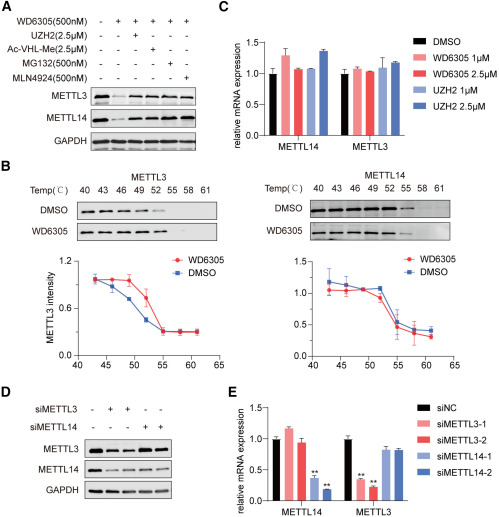
Figure 3. Mechanism of degradation of the METTL3-METTL14 complex (Du W, 2024)
This study confirmed that WD6305 is a potent and selective PROTAC degrader for the METTL3-METTL14 complex. WD6305 inhibits m6A modification and AML cell proliferation and induces apoptosis more effectively than its parent inhibitor. In addition, WD6305 impacts multiple signaling pathways associated with AML development and proliferation. The study reveals PROTAC degradation of the METTL3-METTL14 complex as a potential anti-leukemia strategy and offers an ideal chemical tool for further understanding the function of the METTL3-METTL14 protein.
Reference
Du W, Huang Y, Chen X, et al. Discovery of a PROTAC degrader for METTL3-METTL14 complex[J]. Cell Chemical Biology, 2024.
