Recently, researchers have found that a Middle East respiratory syndrome coronavirus vaccine has shown efficacy in human trials, which may help to develop a vaccine against the new virus. Professor Marylyn Addo, a DZIF scientist and author of the study’s newsletter and head of the infectious diseases department at Hamburg-Eppendorf University Medical Center in Germany, explained: “The results of the vaccine trials are important and hopeful for the development of a vaccine against novel coronavirus SARS-CoV-2. The development of the MERS vaccine provides a basis for us to rapidly develop a vaccine against novel coronavirus in DZIF.”
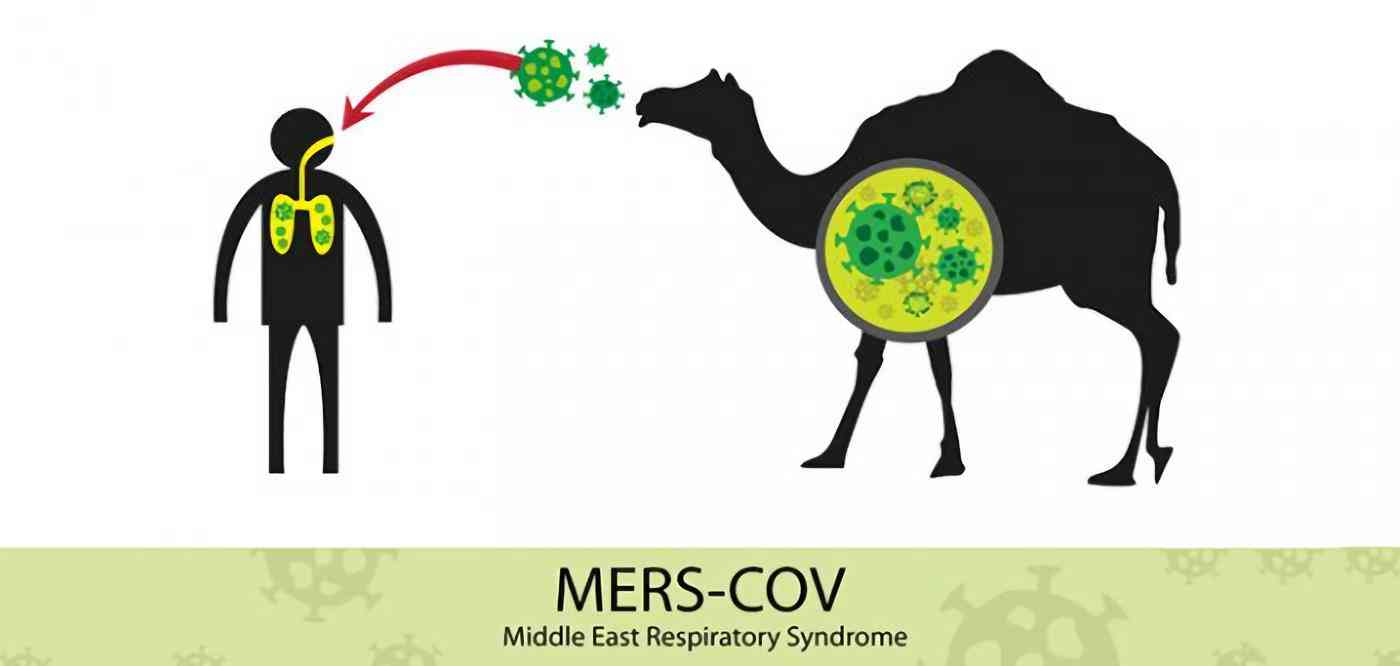
The first discovery of the Middle East Respiratory Syndrome coronavirus in 2012 was listed as a blueprint for pathogens in the World Health Organization (WHO) which are considered to pose a particular threat to public health. This virus can be transmitted from single camel to humans and can also spread among humans. Infection with this virus causes respiratory diseases and mortality rates rise to 35%. Globally, 27 countries have found nearly 2500 cases of MERS cases, including Saudi Arabia’s largest cases. So far there have been no effective vaccines against MERS and no specific drugs.
Candidate vaccine MVA-MERS-S
Addo explained: “2014, together with DZIF partners, we started developing a vaccine against the Middle East respiratory syndrome coronavirus, preparing for larger viruses in the future.” The vaccine is based on an attenuated virus (MVA: modified vaccinia virus-Ankara virus) which was used in a campaign against smallpox and has now been modified to contain protein components from MERS coronavirus. This recombinant vaccine, also called MVA-MERS-S, is a viral vector vaccine that aims to improve immunity against MERS coronavirus. Professor Gerd Sutter of Munich University developed this vaccine in collaboration with researchers at the University of Marburg and the Medical Centre at Rotterdam in Rotterdam. MVA vectors are now developing bases for novel coronavirus vaccine vaccines.
Vaccine trials
A total of 23 healthy volunteers received two MVA-MERS-S vaccinations at intervals of four weeks. The purpose of this trial is to answer three questions: is the experimental vaccine MVA-MERS-S well tolerated? Is it safe to use it on the human body? Does it trigger humoral and cellular immune responses? That is, can it products antibodies and T cells that can prevent MERS-CoV infection or inhibit the progression of the disease?
The most important discovery
“The tolerance, safety, and immune response of candidate vaccines are very promising,” explained Dr. Till Koch, one of the first authors of the trial. The vaccine was well tolerated. Local side effects (i.e. pain at the injection site, mild erythema and fever) were the most common, occurring in 69% of subjects. There were no serious side effects. “After the second injection of MVA-MERS-S, 87% of the subjects showed antibody production and T cell response,” concluded co-lead author Dr. Christine Dahlke.
Professor Stephan Becker expressed satisfaction, “these results suggest that this new vaccine may be used for future outbreaks of Middle East respiratory syndrome.” The subjects’ antibody responses were investigated in his laboratory at the University of Marburg. At DZIF, Stephan Becker is responsible for coordinating “new infections” in the field of research and actively participating in all vaccine projects.
The way of developing Vaccine
Next, the Ib phase trial, funded by the CEPI (Alliance for innovative Epidemiology Preparedness), will test the vaccine in 160 subjects in Hamburg and Rotterdam. The German infection Research Center will use the results of this trial to start developing a new coronavirus vaccine as soon as possible. The scientists will use the same viral vector (MVA), in which they will insert a SARS-CoV-2 spike protein to replace MERS-CoV’s spike protein.
