NASH Target Development Service for IgG-Enhanced Bovine-Derived Colostrum
Bacterial lipopolysaccharide (LPS) and endogenous intestinal bacterial endotoxin are important cofactors involved in the pathogenesis of liver injury in non-alcoholic steatohepatitis (NASH). IgG-enhanced bovine colostrum specific for LPS has been shown to reduce liver injury and provide a potential therapeutic strategy for the treatment of NASH. As a first class and the undisputed world-leader of NASH drug discovery, Creative Biolabs has a "one-stop" drug development service platform, which combines its own advantages to provide customers with a range of technical services about NASH.
Introduction of Bovine-Derived Colostrum
Bovine colostrum (BC) is milk from lactating mammals that is secreted during the first 72 h after birth and its composition differs markedly from that of milk produced later in lactation. BC contains abundant bioactive components that provide immunoglobulins (Igs) and essential and non-essential nutrients for newborns. Among them, Igs account for approximately 5% of the total content and are the main immune components of the acquired immune system. BC is also rich in cytokines and other immune agents, providing antibacterial, bactericidal, antiviral, anti-inflammatory and immunomodulatory protection against infection. BC can also serve as an easy and safe method for generating antigen-specific antibodies, and it can also serve as a source of immunological adjuvants, both of which have been shown to activate innate systems. In brief, the main actions of BC include antibacterial effects and a modulation of the immune response.
IgG-Enhanced Bovine-Derived Colostrum for NASH Treatment
Insulin resistance and metabolic syndrome are chronic inflammatory conditions that lead to hepatic injury and NASH. Chronic low-grade inflammation involves endotoxin derived from the intestinal flora. Lipopolysaccharide (LPS) and endogenous intestinal bacterial endotoxin are considered to be an important cofactor that mediates the pathogenesis of liver injury in NASH. In addition, obesity is also closely related to NASH. Fatty livers are susceptible to injury induced by secondary inflammatory stress, including injury caused by endogenous intestinal LPS. Natural killer T (NKT) cells play an important role in the susceptibility of fatty liver to LPS. NKT cells are part of the innate immune system that regulates local T helper cell type 1 (Th1) and other anti-inflammatory Th2 cytokines produced by monocytes in the liver.
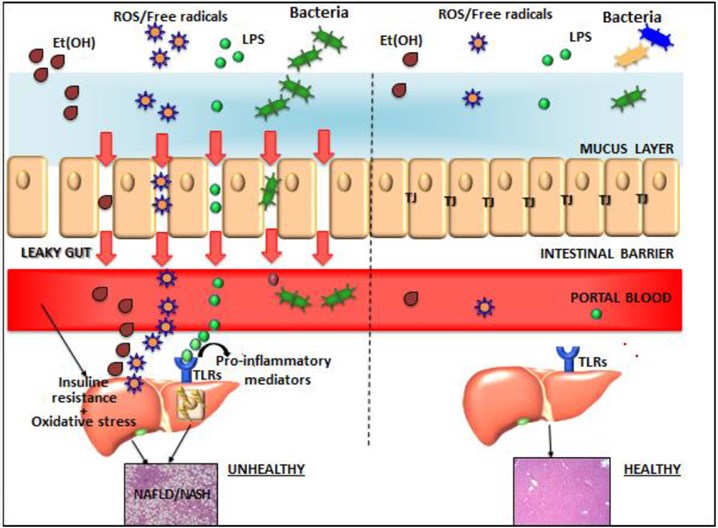 Fig.1 Gut-liver axis components in normal conditions and in non-alcoholic fatty liver disease (NAFLD). (Poeta, 2017)
Fig.1 Gut-liver axis components in normal conditions and in non-alcoholic fatty liver disease (NAFLD). (Poeta, 2017)
At present, Imm124-E, prepared from cows immunized with a vaccine containing an extract of the surface antigens from the most common varieties of enterotoxigenic E. coli, shows a clear improvement in insulin resistance and to alleviation of related liver injury in NASH patients. Moreover, an IgG-enhanced bovine-derived colostrum (IgG-LPS), prepared from cows immunized against LPS from intestinal bacteria (E. coli), can promote the activity of NKT cells and alleviate liver injury, chronic inflammatory, and improve insulin resistance, fatty liver disease, thereby leading to reduced liver injury in NASH. These effects may be attributed to the specific activity of the IgG fraction against the human intestinal microorganisms used to produce the hyperimmune colostrum in cows. Thus, hyperimmune BC preparations could allow production of a safe treatment for NASH.
In addition to NASH-related services, Creative Biolabs also provides drug design and antibody development services (including Phage Display & Antibody Library Services, Antibody Analysis Services, Antibody Engineering Services) based on other diseases. Our service platform is a cost-effective and effective option for you to accelerate the development of drug targets. If you would like more information, please contact us.
Reference
- Poeta, M.; et al. Gut-liver axis derangement in non-alcoholic fatty liver disease. Children. 2017, 4(8): 66.
 For Research Use Only.
For Research Use Only.