

As a leading service provider in the field of T cell antigen connector (TAC) engineered T cell immunotherapy development, Creative Biolabs focuses on designing and developing safe and more effective T cell therapies for the treatment of various diseases. We offer a full range of TAC-related services to help our worldwide customers shorten the drug discovery time and lower the cost of drug development. With our proven competencies and regulatory expertise, we are therefore confident in designing, developing, testing, and manufacturing numerous TAC-T cells to suit your special needs in any immunotherapy project.
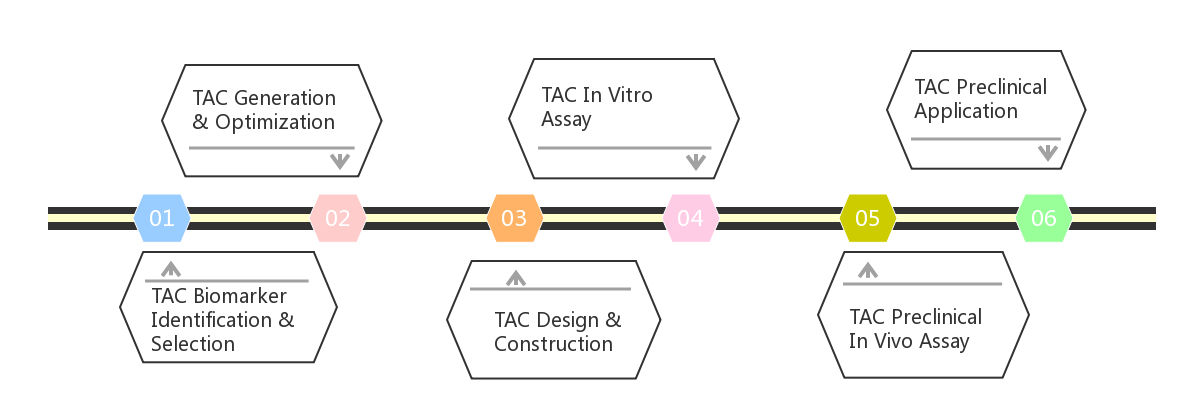 Fig.1 One-stop Solution of TAC Engineered T Cell Development Services.
Fig.1 One-stop Solution of TAC Engineered T Cell Development Services.
Our process for manufacturing off-the-shelf CellRapeutics™ TAC products begins with the collection of white blood cells from healthy human blood. The collected cells are then screened, tested, and then the T cells are isolated and frozen to form a stock of healthy donor cells for manufacturing.
Next, T cells are engineered to express TAC-T cells that recognize certain cell surface proteins expressed in hematologic or solid tumors. TAC-001 is our primary research product targeting BCMA for the treatment of multiple myeloma. Our next most advanced product is TAC-002 targeting CD19, which is currently being investigated for the treatment of acute lymphoblastic leukemia (ALL). The final step in the process involves the optimization of TAC engineering to achieve the full therapeutic effect.
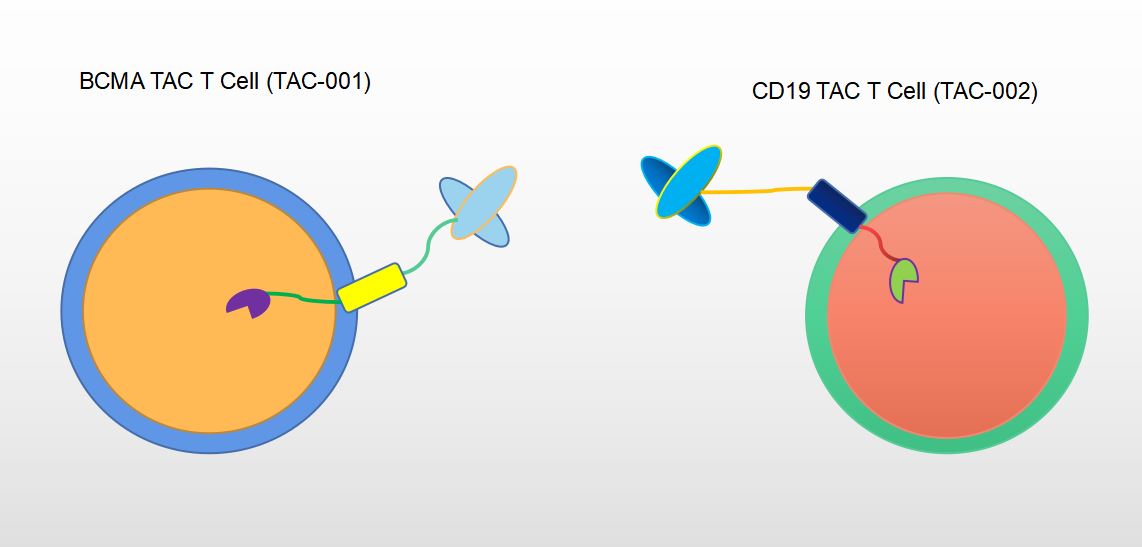 Fig.2 Our CellRapeutics™ Pipeline Targets BCMA (TAC-001) and CD19 (TAC-002).
Fig.2 Our CellRapeutics™ Pipeline Targets BCMA (TAC-001) and CD19 (TAC-002).
In general, successful TAC-T cell development depends on the affinity of the target (usually mAbs) binding region. As a result, the use of mAbs as TAC lead candidates have been maturing in our company. We have used more than 20 mAbs to construct TAC-T with remarkable results. Why choose us?
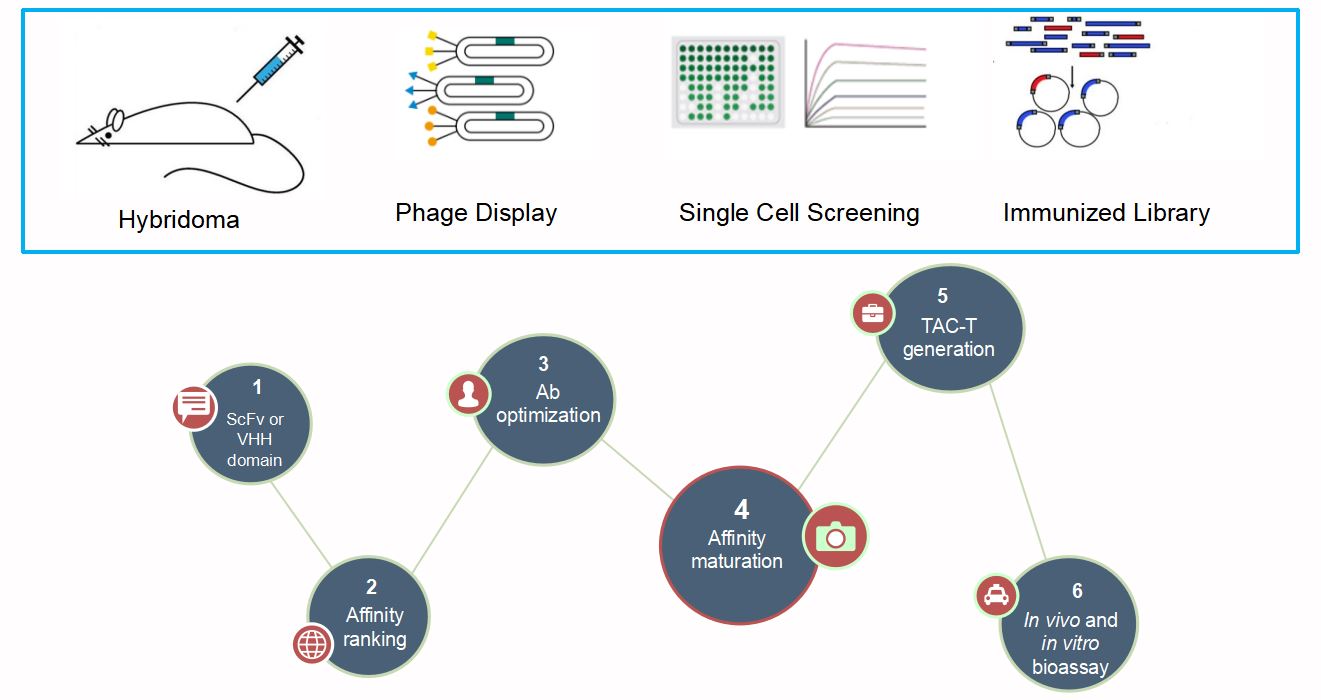 Fig.3 TAC Leads Discovery.
Fig.3 TAC Leads Discovery.
Currently, we are developing TAC modification techniques to improve the efficacy and safety of TAC-T cells in the treatment of solid tumors. Specifically, mimicking the natural T-cell activation pathway leads to serial tumor cell killing, strong clonal expansion, and long-term T-cell persistence while greatly reducing toxicity and increasing cell tolerance. Meanwhile, a majority of approaches to enhance the affinity of TAC-T cells to their target have been also made to trigger cell activation in an MHC-independent manner and without causing any toxicity.
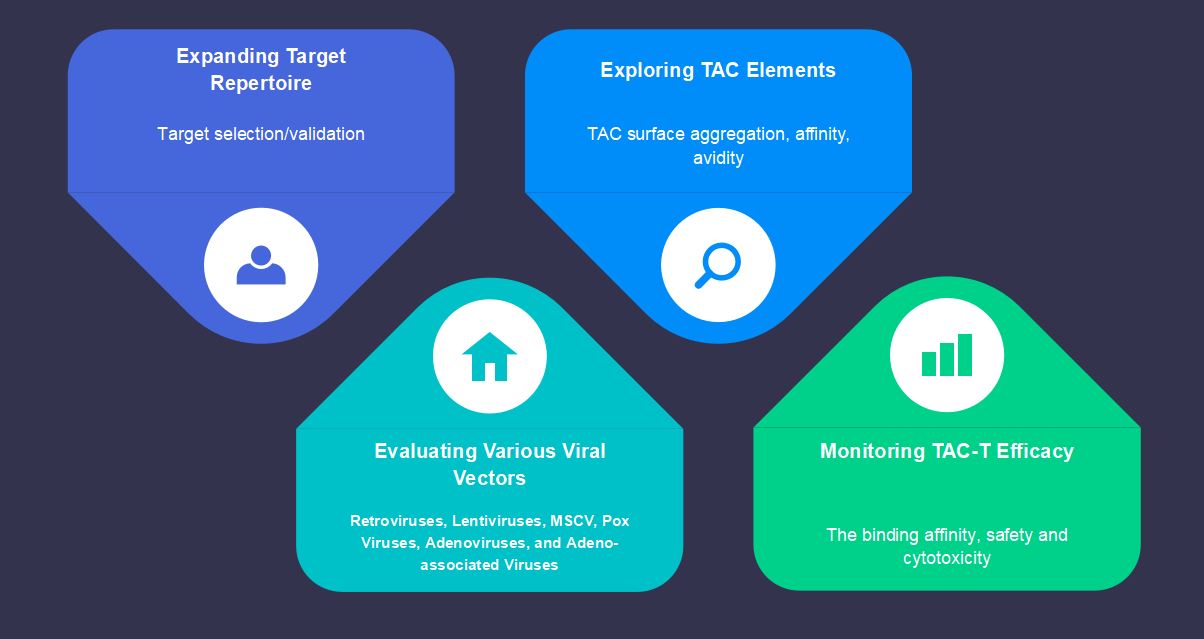 Fig.4 Creative Biolabs is generating the future of the CellRapeutics™ Platform.
Fig.4 Creative Biolabs is generating the future of the CellRapeutics™ Platform.
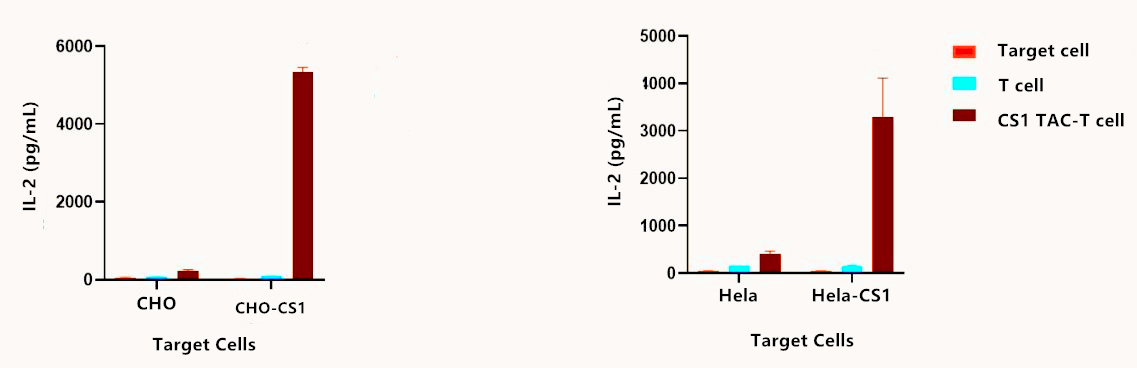 Fig.5 Analysis of cytokine IL-2 secretion in BCMA CS1-TAC.
Fig.5 Analysis of cytokine IL-2 secretion in BCMA CS1-TAC.
Creative Biolabs provides one-stop solutions for TAC-engineered T-cell development services. With a large and well-equipped scientist team, we are dedicated to collaborating with researchers around the world to meet customer specifications. Our clients have direct access to our staff and prompt feedback on their inquiries. If you are interested in our services, please contact us for more details.
For Research Use Only