Antibody-Oligo Conjugates (AOCs)
Overview of AOCs
Oligonucleotides (ONs) are a type of precise payload because of their potential to halt protein production by focusing on certain genes. Despite their selectivity, ONs have several problems, including short serum stability, limited membrane permeability, and a lack of tissue selectivity. Antibodies, with their prolonged half-life, ability to selectively deliver medicines inside cells, and targeting properties, are good partners for ON delivery. Antibody-oligonucleotide conjugates (AOCs) combine the great precision of ONs and the delivery capability of antibodies, have therefore become an important milestone in the development of numerous biotechnological applications, including imaging, detection, and therapeutics (targeted siRNA/antisense delivery).
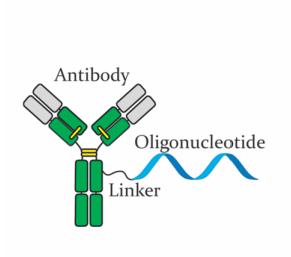 Fig 1. The structure of antibody-oligonucleotide conjugates.1
Fig 1. The structure of antibody-oligonucleotide conjugates.1
Applications of AOCs
AOCs can be applied to medication delivery, immunotherapy enhancement, and biomarker detection to improve treatment efficacy and diagnostic sensitivity. They have been utilized for therapeutic purposes by carrying ASO or siRNA payloads and as a pretargeting component for both radionuclide therapy and imaging applications. In these circumstances, the antibody targets the location of interest, where the coupled ONs may operate as a gene-silencing or pretargeted therapeutic agent. Figure 1 depicts the various applications of AOCs.
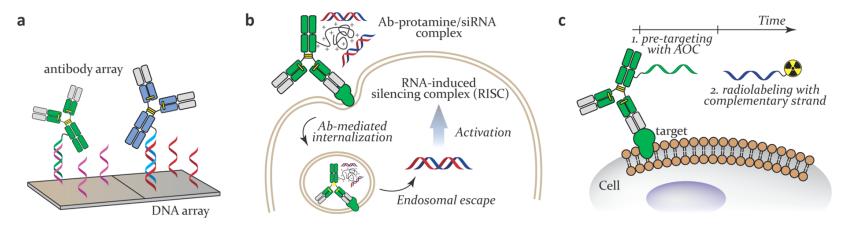 Fig 2. Schematic representation of AOC using (a) antibody arrays; (b) therapeutic; and (c) pretargeting applications.1
Fig 2. Schematic representation of AOC using (a) antibody arrays; (b) therapeutic; and (c) pretargeting applications.1
AOC Synthesis Services
The coupling mechanism between ONs and antibodies significantly impacts the release of ONs medicines, thereby influencing medicinal efficacy. Creative Biolabs offers four synthetic methods as illustrated in Fig 3.
- Ion Interactions-Based Conjugation
We achieve coupling by modifying the antibody through fusion or alteration to attach a polycationic fraction, creating interactions between positive and negative charges that result in coupling with the oligonucleotide backbone. This method applies to a wide spectrum of ONs because it offers simplicity and flexibility for conjugating various oligonucleotide sequences with a single polycationic protein. It enables the evaluation of different gene knockdowns using the same antibody and assessing their activity in cells. Additionally, the polycationic complex acts as a lysosomal escape agent, facilitating the entry of oligonucleotides into the cytosol by inducing osmotic swelling. However, its main drawback lies in the reversible nature of ionic interactions, potentially leading to instability, especially under pH or salt concentration changes.
- Avidin-Based Conjugation
We exploit the strong interactions between biotin-ONs and avidin, or utilize neutravidin as a bridging group for antibodies, to produce AOCs. One advantage of this approach is in vivo stability of the resulting conjugates. It offers the potential to target gene knockdown in specific tissues without eliciting systemic effects, as seen in knockout mouse models, presenting a more accessible and broadly applicable method for modeling disease states. Nevertheless, its universality may be limited compared to Ion interactions-based methods due to the requirement for multiple procedural steps.
- Direct Conjugation
We use a linkable group that is added to the ON and immediately attached to the antibody's lysine, cysteine, or synthetic amino acid. The advantage of this technique over bigger protamine or avidin complexes is that the connections are smaller and have a lower impact on the overall conjugate. Despite the absence of lysosomal escape agents, this may result in a gradual release of the ON from the lysosome, potentially affecting the AOC's efficacy. Nevertheless, due to their cost-efficiency and rapidity, they are expected to become the preferred method for generating AOCs in the future.
- Hybridization Conjugation
We also use double-stranded ONs (such as binding a single-stranded ON to an antibody and then hybridizing the complementary strand) to produce AOCs. The length of the ONs plays a crucial role in this hybridization process. If the ON sequence is too short, the resulting duplex may lack stability in plasma, whereas excessive length could lead to the formation of unwanted secondary structures. An instance involves utilizing cetuximab as a targeting agent to deliver doxorubicin, which can penetrate cells by intercalating within the double-stranded ONs. The precision and selectivity of hybridization enable the construction of precise structures and the integration of chemotherapeutics. While this hybridization technique proves highly effective in diagnostic applications, its application to AOCs targeted for disease poses greater challenges particularly when considering the overall cost of the process.
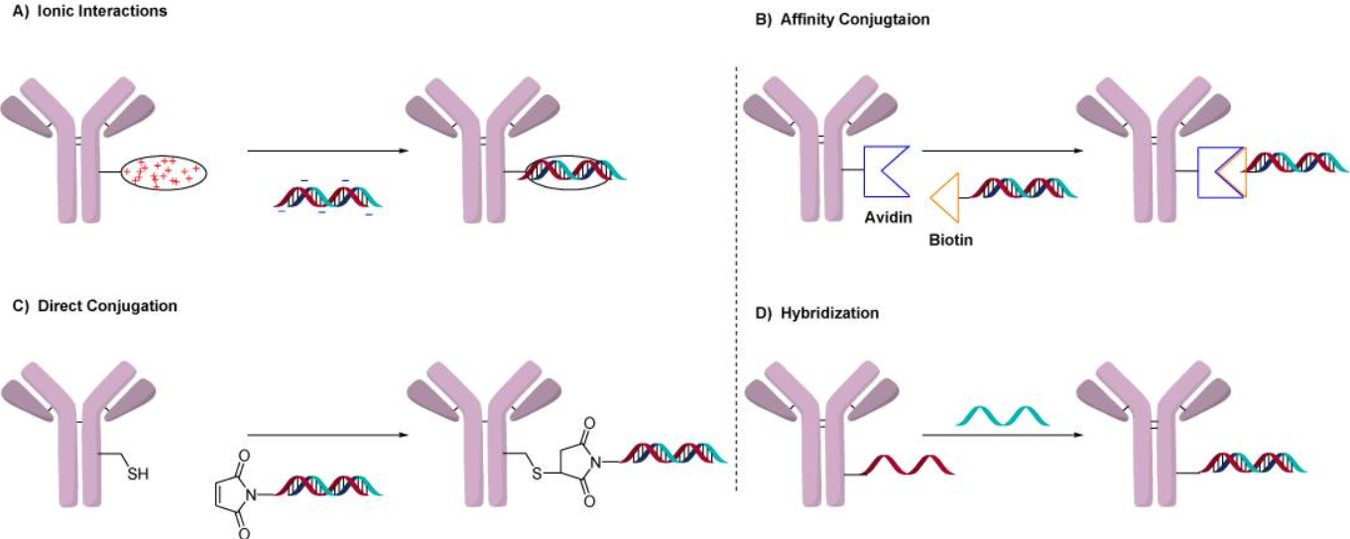 Fig.3 The four general methods used to prepare AOCs.2
Fig.3 The four general methods used to prepare AOCs.2
Creative Biolabs offers extensive AOC synthesis services to customers globally. With advanced synthesis technologies of siRNA, ASOs, and antibodies, we can assure the effectiveness of AOCs. Features of our AOC services include:
- Customized services, we assist customers in tailoring service plans to meet their specific requirements. This includes helping you choose or design suitable coupling strategies.
- High coupling efficiency, our AOCs exhibit a minimum efficiency of 90%, ensuring no more than 10% unconjugated antibody, and its coupling efficiency is conducted using HIC-HPLC.
- High-purity products, we deliver products of exceptional purity through the utilization of gravity columns for the purification of AOCs, followed by assessing purity using SEC-HPLC characterization techniques.
- High-output, our production system boasts an impressive yield, reaching nearly 80%.
- Cost-effective and fast delivery.
For more detailed information, please feel free to contact us.
References
- Dovgan, Igor, et al. "Antibody–oligonucleotide conjugates as therapeutic, imaging, and detection agents." Bioconjugate Chemistry 30.10 (2019): 2483-2501.
- Dugal-Tessier, Julien, Srinath Thirumalairajan, and Nareshkumar Jain. "Antibody-oligonucleotide conjugates: a twist to antibody-drug conjugates." Journal of Clinical Medicine 10.4 (2021): 838.
