Antibody-siRNA Conjugates (ARCs)
Overview of ARCs
Antibody-small interfering RNA (siRNA) conjugates (ARCs) represent an innovative class of biomolecules merging the specificity of antibodies with the gene-targeting capabilities of siRNA. This strategy aims to exploit the precise targeting of antibodies and the gene-silencing properties of siRNA to deliver therapeutic payloads to specific cell types or subcellular compartments, thereby suppressing the expression of target genes. By combining these two elements, ARCs overcome the limitations of poor siRNA targeting and short in vivo circulation time, offering a promising solution for targeted gene silencing and improved therapeutic efficacy.
ARCs Synthesis Service
ARC system consists of three main components: antibodies, siRNAs, and linkers. The key technology for developing ARCs relies on the successful conjugation of antibodies and siRNAs. Creative Biolabs has devised multiple methodologies for coupling siRNAs with antibodies, employing non-covalent interactions or covalent binding to lysine or cysteine residues within the antibody structure, as depicted in Figure 1. This innovative approach holds promise for treating a wide range of diseases by leveraging the specificity of antibodies and the gene-silencing capabilities of siRNAs.
- Non-covalent-based conjugation
Panel a depicts the assembly of ARCs through electrostatic non-covalent interactions, commonly achieved by combining protamine and nona-arginine to form a robust complex. This method is straightforward and does not require complex chemical modifications or specialized linking agents. However, a notable challenge lies in the lower stability associated with this method.
- Sulfhydryl and maleimide reaction-based conjugation
Panel b demonstrates the chemical conjugation of siRNA with the introduced cysteine residue of the IgG antibody using N-Succinimidyl S-acetyl thioacetate (SATA) reagent through a thiol-maleimide reaction. This method enables site-specific conjugation and facilitates the production of large-scale ARCs.
- CPP-based conjugation
Panel c illustrates the use of cationic cell-penetrating peptides (CPPs) for conjugation. To address the challenge of low intracellular uptake efficiency of siRNA due to its negative charge, cationic components such as CPPs are employed to neutralize the charge of siRNA, facilitating its intracellular delivery and enhancing endosomal escape. Moreover, some cationic peptides have demonstrated the ability to disrupt endosomal membranes, aiding in cargo release from the endosome. This CPP-based ARC conjugation strategy has shown considerable success in silencing mRNA in both muscle and tumor cells.
- Fab-based conjugation
Panel d presents the chemical conjugation of siRNA to the C-terminus of antigen-binding fragments (Fabs). Fabs, devoid of an Fc domain, present several significant advantages compared to full-length monoclonal antibodies (mAbs), including enhanced tissue penetration, increased tolerance, and decreased likelihood of immune system activation. Fab-based ARCs have been used to treat rare muscle diseases, such as DM1, Duchene muscular dystrophy, and facioscapulohumeral muscular dystrophy.
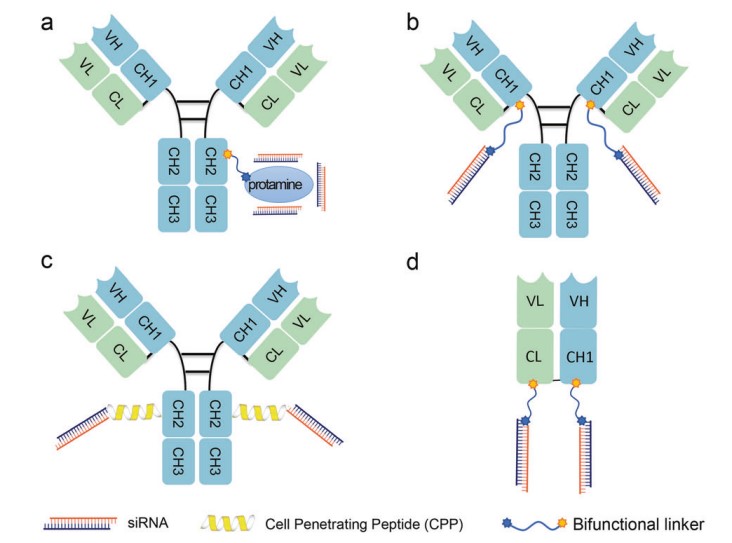 Fig.1 The main coupling methods of ARCs.1
Fig.1 The main coupling methods of ARCs.1
Applications of ARCs
ARCs offer promising applications in treating a spectrum of diseases, including breast cancer, prostate cancer, colon cancer, multiple myeloma (MM), HIV, and leukemia. As illustrated in Figure 2, a linker consisting of a cell-penetrating peptide (CPP) and a substrate peptide (SP) specific to solid tumors was employed to attach ARCs, enabling targeted delivery of siRNA into tumor cells. Upon hydrolysis of the substrate peptide linker by enzymes on the tumor cell surface, the siRNA-CPP payload was released from the ARCs. By harnessing the cell-penetrating capability of CPP, the liberated siRNA-CPP efficiently penetrated targeted cells, resulting in amplified gene silencing efficacy in both in vitro and in vivo settings. Upon intravenous administration, the engineered ARCs achieved a notable 66.7% reduction in EGFP (Enhanced Green Fluorescent Protein) expression in a murine tumor model, demonstrating their safe and efficient delivery capability. These advancements herald a new era in disease therapeutics, with ARCs poised to address unmet needs across various challenging conditions.
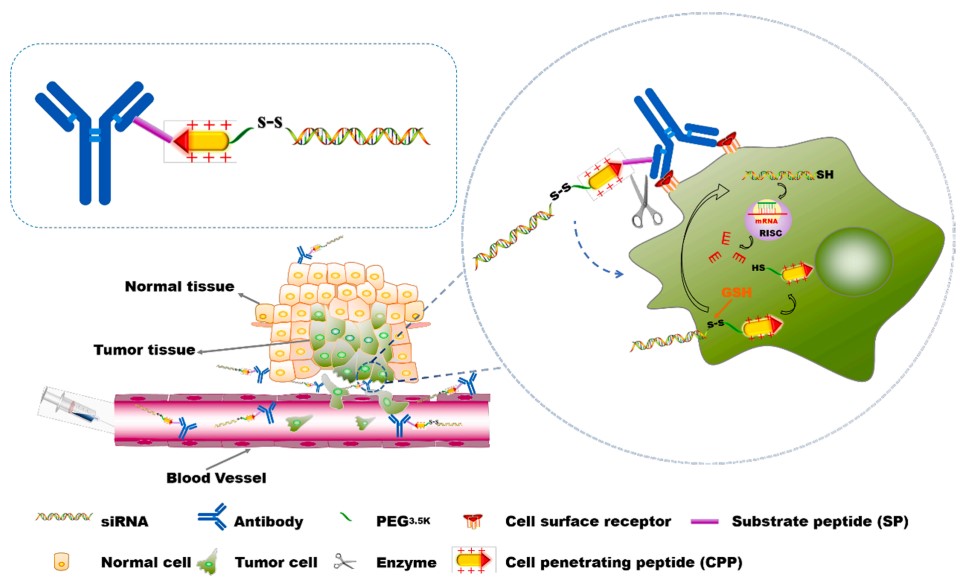 Fig.2 Schematic illustration of CPP-ARCs as a delivery platform to deliver siRNA into targeted tumor cells.2
Fig.2 Schematic illustration of CPP-ARCs as a delivery platform to deliver siRNA into targeted tumor cells.2
Creative Biolabs is committed to providing high-quality ARC synthesis services to customers globally. With RNA-based therapeutics like ARCs holding immense potential, we continuously innovate to develop diverse ARC systems. Leveraging our extensive expertise in siRNA synthesis and cutting-edge technology platforms for antibody development, our AOC services boast the following features:
- Highest quality products and excellent customized services.
- Strict quality control.
- One-stop-shop service and faster delivery.
- Best after-sale services and cost-saving.
For further information, please contact us.
References
- Jin, Shijie, et al. "Emerging new therapeutic antibody derivatives for cancer treatment." Signal Transduction and Targeted Therapy 7.1 (2022): 39.
- Yu, Zhili, et al. "Antibody-siRNA conjugates (ARCs) using multifunctional peptide as a tumor enzyme cleavable linker mediated effective intracellular delivery of siRNA." International Journal of Pharmaceutics 606 (2021): 120940.
