Lipid Nanoparticle (LNP)
Overview of LNPs
Lipid nanoparticles (LNPs) have emerged as highly effective nano-delivery systems, facilitating the efficient transport of various therapeutic agents, notably mRNA vaccines for combatting infectious diseases like COVID-19. LNPs typically comprise four components, each playing a crucial role as depicted in Fig.1. These constituents contribute to the formation of stable, uniform nanoparticles, enabling efficient encapsulation of diverse nucleic acid cargoes such as DNA, ASOs, siRNA, microRNA, and mRNA.
- Ionizable lipid
Ionizable lipids play a critical role in facilitating the efficient encapsulation of nucleic acid cargo within LNPs, commonly achieving encapsulation rates surpassing 90%. During the formation of nanoparticles, ionizable lipids undergo a positive charge, facilitating their interaction with the negatively charged phosphate backbone of nucleic acid polymers through electrostatic forces, thus promoting the incorporation of nucleic acids into the developing nanoparticle structure. These lipids are pivotal for the successful development of LNPs due to their multifaceted functions. Firstly, the apparent pKa of ionizable lipids regulates effective LNP-mediated transfection. Secondly, ionizable lipids may be crucial for the cellular internalization of LNPs. Lastly, they might also influence the tolerability and immunogenicity of LNPs.
- PEG-lipid
PEG-lipids serve varied functions within LNP formulations and are essential for mRNA delivery. Their quantity in LNP formulations dictates crucial properties such as size and zeta potential, which significantly influence delivery efficacy. Additionally, PEGylated lipids contribute to enhancing stability and reducing LNP aggregation. Additionally, they protect LNPs against uptake by the mononuclear phagocyte system (MPS). While indispensable for LNPs, the immunogenicity of PEG and the presence of anti-PEG antibodies must be carefully considered in the development of LNP-based vaccines and therapeutics.
- Phospholipid
Phospholipids serve as essential "helper lipids" in LNP formulations. They not only provide structural stability to the nanoparticles, thereby improving their biodistribution and enhancing delivery efficacy by facilitating intracellular uptake and cytosolic entry but also, depending on their types, can induce lipid bilayer disruption, promoting endosomal escape. Among the frequently utilized phospholipids is phosphatidylcholine (PC), including variants such as 1,2-distearyol-snglycero-3-phosphocholine (DSPC) and hydrogenated soybean PC (HSPC).
- Cholesterol
Cholesterol plays a pivotal role in LNPs as a crucial component, contributing to enhancing particle stability through its regulation of membrane integrity and rigidity. In LNP formulations, an equimolar amount of cholesterol is typically included relative to endogenous membranes. This balance prevents net efflux or influx, thus preserving membrane integrity.
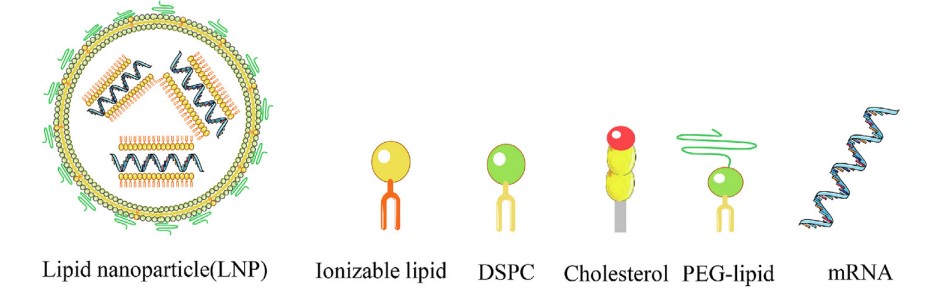 Fig.1 Illustration of LNP and its compositions.1
Fig.1 Illustration of LNP and its compositions.1
Application of LNPs
LNP technology is currently regarded as one of the most forefront non-viral gene delivery systems in clinical practice. They can be used for gene therapy, protein replacement, and vaccine development, as shown in Fig.2.
- mRNA vaccines
mRNA vaccines offer a novel technology for combatting infectious diseases and show promise in cancer therapy due to their ease of manufacture and acceptable immunogenicity. LNPs enable efficient mRNA delivery, reducing toxicity and immunogenicity concerns while achieving in vivo transfection efficiency. Additionally, LNPs are utilized for delivering chimeric antigen receptor (CAR) mRNA, enabling the production of CAR cells, and offering a promising approach to cancer treatment. LNPs facilitate efficient ex-vivo transfection of T cells, eliminating the need for viral vector transduction in chimeric antigen receptor T cell (CAR-T) or T cell receptor-engineered T cell (TCR-T) therapies, thereby enhancing the safety profile of current clinical T-cell therapy. mRNA-LNP-based interventions are presently undergoing validation across various cancer types, including melanoma, lymphoma, hepatocellular carcinoma, colorectal cancer, breast cancer, and more.
- Gene therapy
LNPs are also utilized in preclinical investigations for transporting various therapeutic payloads, including siRNA, mRNA, antisense oligonucleotides, micro-RNA, and DNA, targeting diseases caused by genetic mutations. LNPs serve as a versatile nucleic acid delivery platform, addressing challenges like nucleic acid degradation and limited cellular uptake in gene therapy. Traditional LNPs tend to accumulate in the liver and kidneys, and about 80-90% of LNPs end up in the liver after intravenous injection. For delivery to non-hepatic such as mitochondrial targeting LNP, conjugation with specific ligands is required to enhance LNP targeting. By attaching ligands to the LNP surface, specific target molecule identification and interaction can be achieved. Main conjugation strategies include Direct Assembly, Post-Modification, and Post-Insertion. Direct assembly relies on lipid molecules' amphipathic nature and self-assembly behavior under specific conditions while incorporating drugs or functional molecules to prepare LNPs. This method requires significant modification work and may be more suitable for small molecule ligands due to limitations with larger ligands affecting self-assembly efficiency. Post-modification/post-insertion allows ligand introduction after LNP formation, enhancing the delivery system's effectiveness and precision, offering promise for broader indication development.
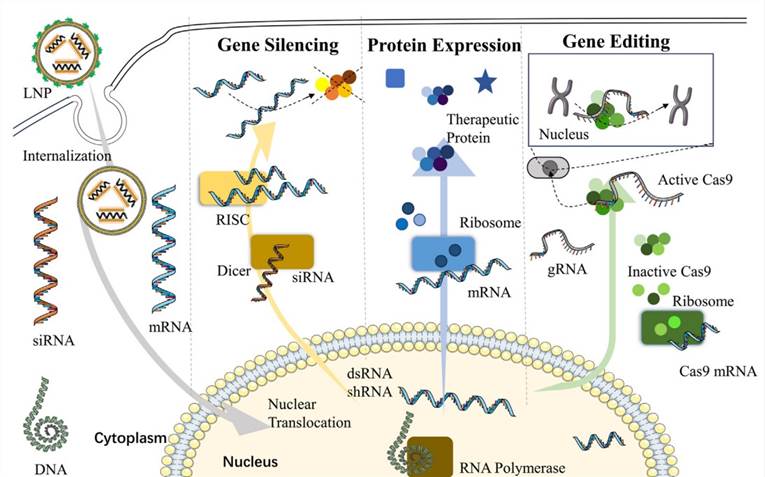 Fig.2 The application of LNP delivery in pharmaceutical therapy.2
Fig.2 The application of LNP delivery in pharmaceutical therapy.2
Our LNP Synthesis Services
Creative Biolabs has a complete IVT mRNA preparation process: from gene synthesis to LNP packaging. There are various production methods for LNPs, including Thin-film hydration, Ethanol injection, T-junction mixing, and Microfluidic mixing. Our LNPs are produced scalability through microfluidic mixing methods, which is the potential platform, that has accomplished efficient and reproducible mRNA-LNPs. The most commonly and effectively utilized techniques for characterizing the size and size distribution of LNPs are ensemble methods such as dynamic light scattering (DLS), static light scattering, and nanoparticle tracking analysis. Our LNPs possess several advantages, including:
- Supports encapsulation of multiple forms of RNA, including mRNA, siRNA, Cas mRNA, and sgRNA.
- High in vitro delivery efficiency.
- High in vivo delivery efficiency and low toxicity.
We also provide rich QC services for LNPs. For more detailed information, please feel free to get a quote, and we will contact you within 24 hours.
Reference
- Yuan, Ye, et al. "Applications of artificial intelligence to lipid nanoparticle delivery." Particuology 90 (2024): 88-97.
