CD133 widely recognized as a cancer stem cell marker associated with drug resistance and cancer relapse, has recently been the focus of a study published in the international journal eLife titled “Identification of CD133+ intercellsomes in intercellular communication to offset intracellular signal deficit.” In this research report, scientists from the University of California and other institutions revealed a unique process where liver cells can share molecules through vesicle exchange, allowing them to proliferate under conditions that would typically inhibit cell growth. The researchers also found evidence suggesting that this process may occur in various types of cancer cells, potentially paving the way for the development of new approaches to address therapy resistance in cancer.
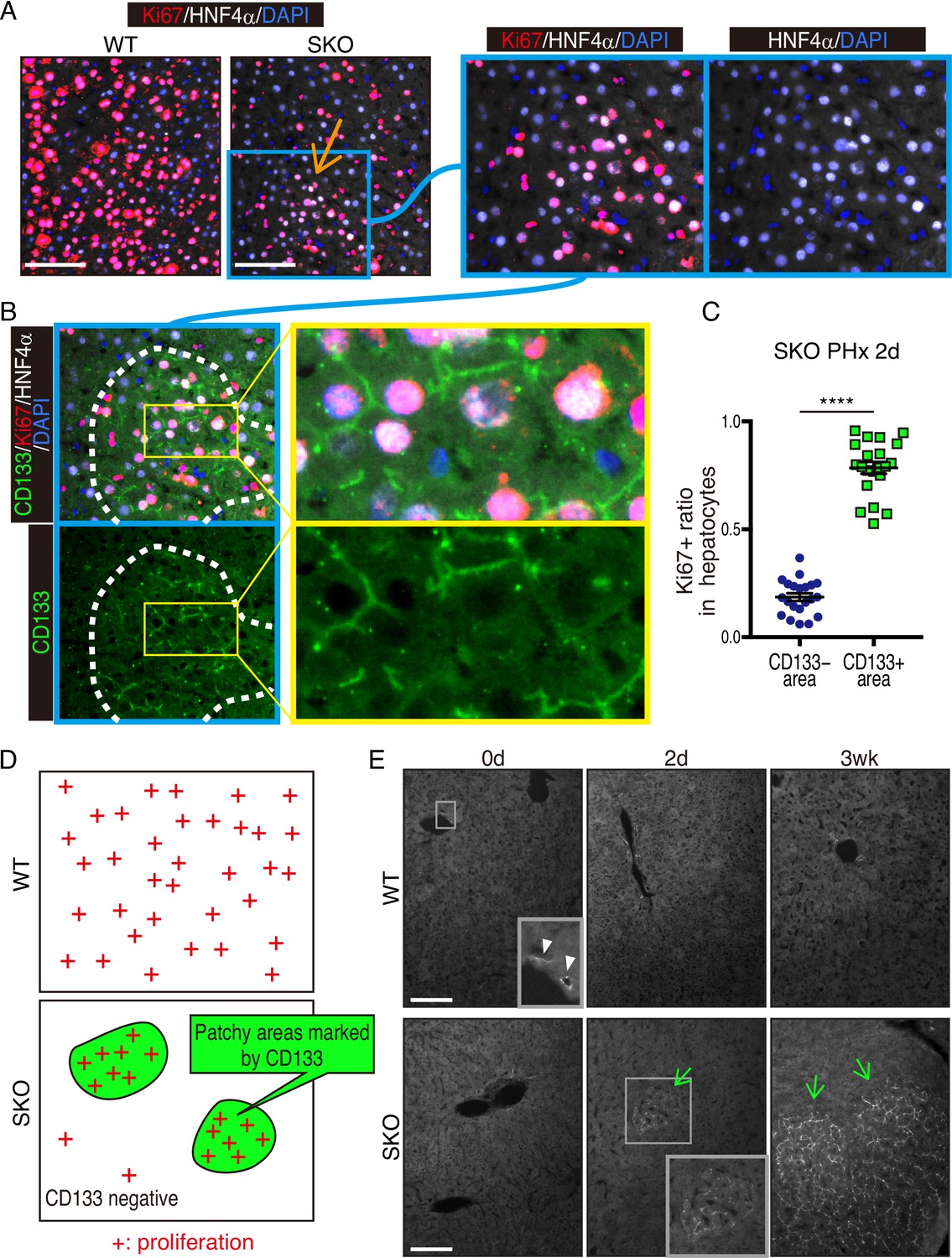
Fig. 1 Identification of CD133+ intercellsomes in intercellular communication to offset intracellular signal deficit (Kaneko, 2023)
Gen-Sheng Feng, the lead researcher, stated that understanding cell proliferation is a fundamental question in cancer research and biomedical science as a whole. While the study focused on the liver due to its incredible regenerative potential, the researchers observed similar vesicle structures in other types of cancer cells. This could indicate a universal mechanism driving cancer cells’ resistance to therapies targeting cell proliferation. The body has numerous molecular processes that help control cell proliferation, regulating it based on the needs in different environments and organs. The liver, with its high regenerative capacity supporting vital detoxification and metabolic functions, serves as an ideal site to study these biological processes.
The researchers noted that liver cells proliferate faster and more efficiently than cells in any other part of the body, making the liver an ideal location to study the control of cell division in biological processes. These processes can go awry in cancer cells, making targeting cell proliferation a promising approach in cancer therapy. In previous studies, Feng and colleagues observed that even when liver cells in the mouse body were genetically engineered to lack a crucial signaling enzyme needed for cell proliferation, a small portion of liver cells still continued to proliferate. This enzyme, called Shp2, helps guide liver cells on when to divide and is a known target for treating various cancers. Shp2 inhibitors are also involved in ongoing clinical trials.
The researchers found that liver cells have a clever way of compensating for the missing enzyme. By clustering together and exchanging tiny protein-wrapped vesicles, liver cells can communicate and share the biochemical substances needed for proliferation, even in the absence of Shp2. While researchers had known that liver cells could regenerate even without Shp2, this study revealed how they achieve this. Although the researchers discovered this in non-cancerous liver cells, they also found evidence that cancer cells might use the same strategy to resist therapy and continue dividing. The vesicles used by liver cells to share molecules were marked by a protein called CD133, and researchers detected abnormally high levels of this protein in various human cancer cells.
Feng stated, “We believe we have discovered a crucial strategy that tumors use to resist therapy.” This discovery proves to be a potent new target for cancer therapy, especially as part of combination therapies aimed at slowing therapy resistance. While these findings will advance the development of new cancer therapies, they also propose a new approach to considering the initiation, progression, and relapse of cancer. CD133, widely considered a marker for cancer stem cells, which are thought to be the culprits behind tumor initiation, might be a transient state that can be induced or even closed. This new observation of the “stemness” or the ability of cancer cells to initiate and restart tumors could provide a fresh perspective on understanding tumor relapse and aid in developing novel anticancer therapies.
Reference
1. Kaneko, Kota, et al. “Identification of CD133+ intercellsomes in intercellular communication to offset intracellular signal deficit.” Elife 12 (2023): RP86824.
