Antibody engineering is revolutionizing immuno-oncology, allowing for the synthesis of synthetic immunity by optimizing various components of antibody structure. Monoclonal antibodies (mAbs) and bispecific antibodies (BsAbs) with enhanced antibody effector functions have been approved for several cancer indications. However, tumors evolve rapidly, often acquiring immune evasion mechanisms, which may include (a) upregulation of CD47 signaling to suppress tumor phagocytosis, (b) interference with complement or NK cell activity, or (c) clonal escape through antigen downregulation. Therefore, novel antibody designs are needed to overcome these tumor evasion mechanisms as the next generation of cancer immunotherapy.
Recently, researchers from the University of Geneva published an article in the journal Nat Commun. titled “Development of ISB 1442, a CD38 and CD47 bispecific biparatopic antibody innate cell modulator for the treatment of multiple myeloma,” demonstrating that ISB 1442, a multispecific antibody, may be used to treat CD38+ hematologic malignancies.
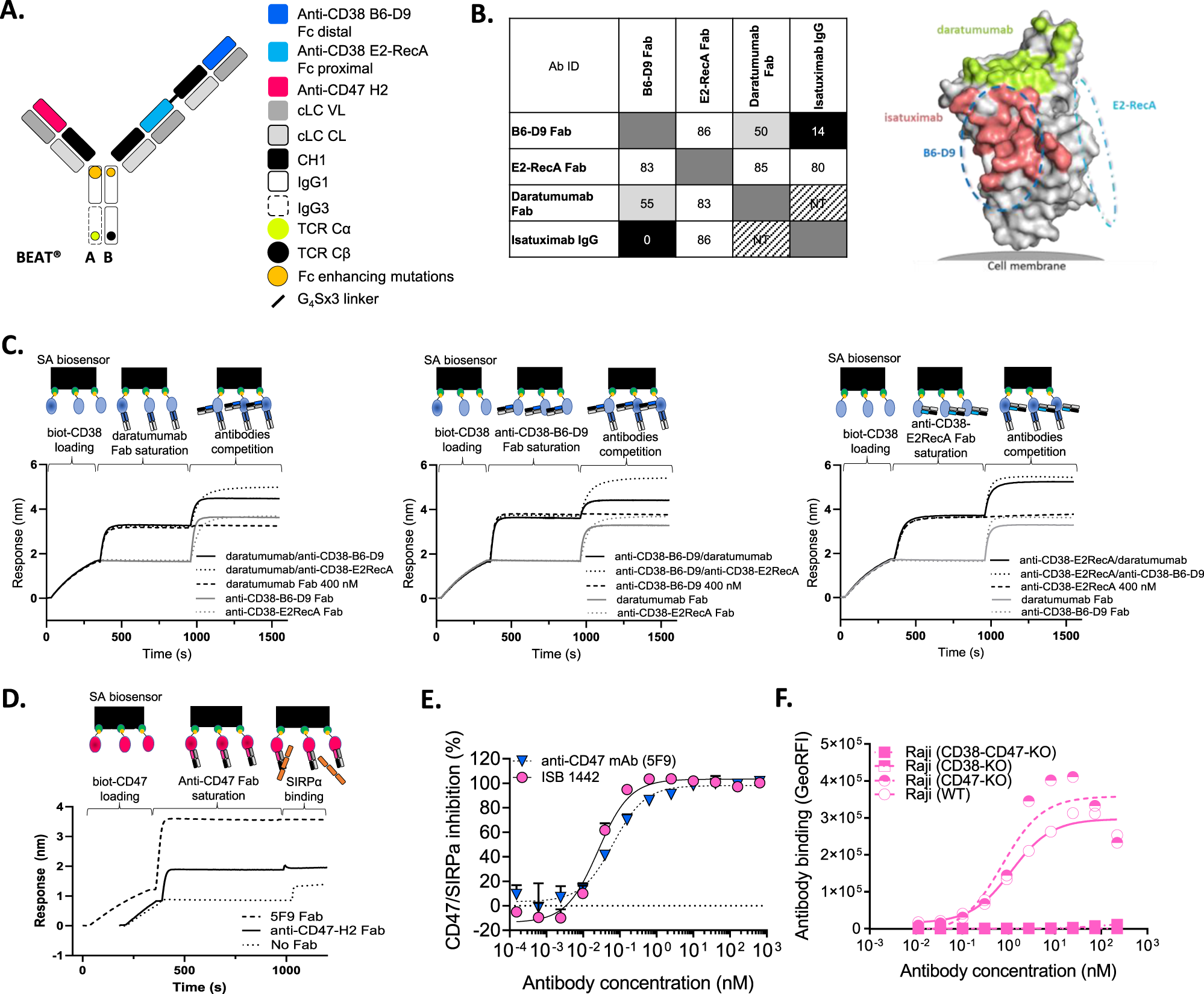
Antibody engineering allows for the customization of therapeutic antibody design and activity to achieve improved efficacy or other favorable clinical characteristics. Here, researchers report the development of ISB 1442, a specificity antibody designed to reconstitute synthetic immunity against CD38+ hematologic malignancies.
ISB 1442 consists of two arms targeting two different epitopes of CD38, which preferentially drive binding to tumor cells and enable induced blockade of proximal CD47 receptors on the same cell, while preventing binding of targeted non-tumor cells to healthy cells. The Fc portion of ISB 1442 is designed to enhance complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and antibody-dependent cellular phagocytosis. Thus, ISB 1442 represents a CD47-BsAb fusion with dual-site targeting of tumor-associated antigens and engineered enhanced antibody effector functions to overcome potential resistance mechanisms hindering monospecific anti-CD38 antibody therapy for multiple myeloma. ISB 1442 is currently in Phase I clinical trials for relapsed/refractory multiple myeloma.
In summary, this study describes the application of BEAT® bispecific antibody technology in the generation of ISB 1442, a bispecific biparatopic antibody capable of inducing synthetic immunity through multiple effector mechanisms while bypassing several tumor evasion mechanisms that weaken the activity of monospecific antibodies. ISB 1442 is envisioned as the next-generation innate immune cell modulator for CD38+ hematologic malignancies and is currently in Phase I clinical trials for RRMM.
What We Do
Creative Biolabs has grown into a recognized world leader in bispecific antibody (bsAb) discovery, engineering, production, and analysis, and provide recombinant anti-CD19 & CD47, anti-CD20 & CD47, and anti-MLSN & CD47 bispecific antibodies in several formats including but limited to Appended IgGs, Tandem scFv, Tandem Fab, Diabody, and TandAb.
Reference
1. Grandclément, C., et al. “Development of ISB 1442, a CD38 and CD47 bispecific biparatopic antibody innate cell modulator for the treatment of multiple myeloma.” Nature Communications 15.1 (2024): 2054.
