Antiretroviral therapy can suppress HIV-1 replication, but the persistence of long-lived resting memory CD4+ T cells harboring latent pre-viral HIV-1 DNA prevents a cure. To date, clinical interventions based on Latency Reversal Agents (LRAs) have not achieved a significant reduction in the latent reservoir, despite observed increases in plasma and cell-associated viral RNA. One method to improve the cell-mediated clearance of HIV-1 infected cells is the use of bispecific antibodies, with immune therapies targeting HIV-1 infected cells primarily focusing on recruiting CD8+ T cells. However, continuous exposure to HIV-1 antigens may lead to CD8+ T cell exhaustion, and several LRAs have been shown to inhibit the cytolytic potential of CD8+ T cells. Furthermore, cytokine release syndrome has occurred in some clinical trials involving T cell-engaging bispecific antibodies, raising safety concerns.
Recently, Professor Nathan L. Board and his team from the Department of Medicine at the Johns Hopkins University School of Medicine in Baltimore, Maryland, USA, reported on two anti-CD16 and HIV-1 envelope protein (Env) single-chain dual antibodies (scDbs), aiming to enhance HIV-1 specific NK cell activity. This suggests that HIV-1 specific scDbs warrant further evaluation as a potential therapy for clearing the latent reservoir.
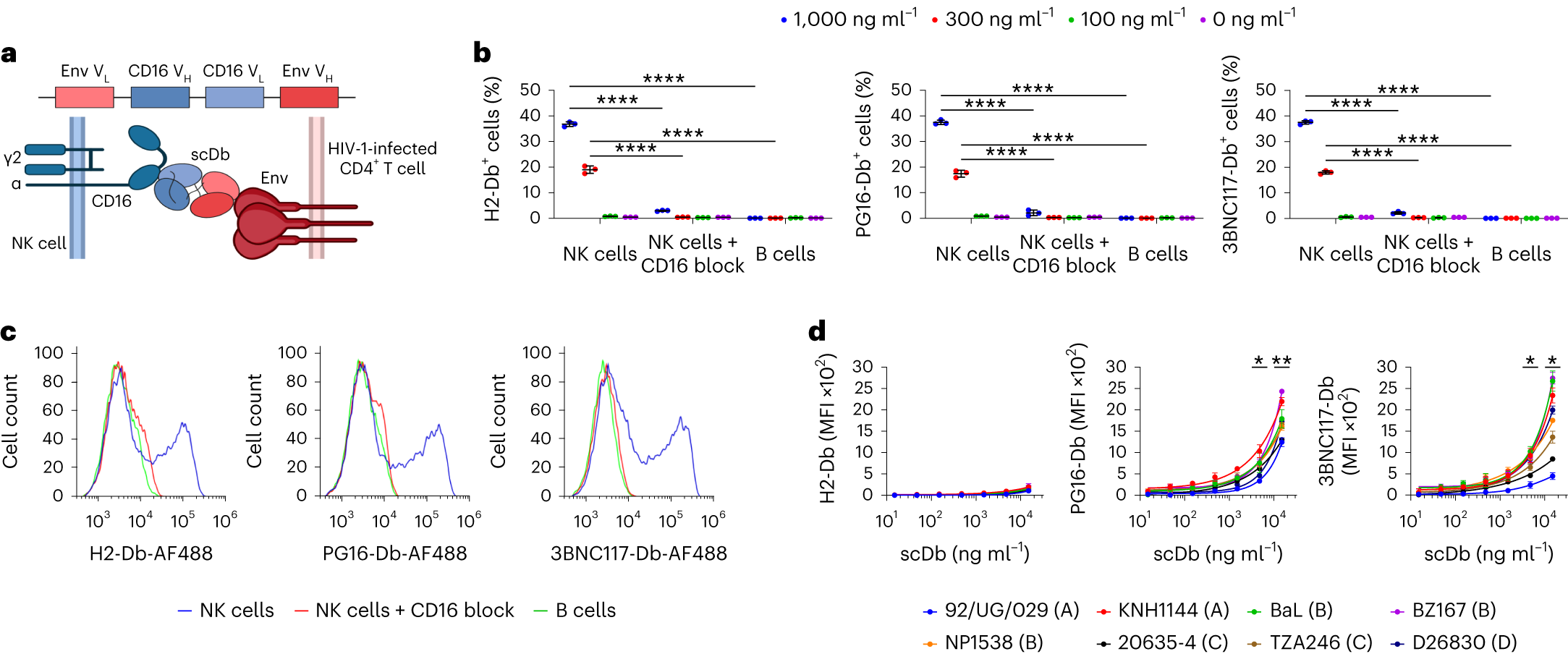
The findings indicate that single LRA treatment only led to a modest increase in HIV-1 caRNA transcripts, with the surface density of Env being a critical factor in the elimination of infected cells by other Env-specific immune therapeutic approaches, including those based on bNAb-CAR T cells. Multiple participants were able to effectively clear complete viral reservoir cells, suggesting that PMA + I treatment could induce sufficient Env expression for scDb-mediated clearance. PG16-Db and 3BNC117-Db target non-overlapping Env epitopes, and combining these drugs resulted in a slightly higher average clearance rate of reservoir cells than clearance mediated by a single scDb at the same concentration. scDbs can engage multiple cytolytic immune cell types, complementing the limitations of CD8 T cell-based bispecific antibodies. The shorter half-life of scDbs in humanized mice, possibly due to the absence of an Fc domain—which prolongs the half-life of conventional monoclonal IgG therapies through interaction with neonatal Fc receptors—highlights a potential area for improvement.
In summary, PG16-Db and 3BNC117-Db merit further evaluation as therapeutic interventions to reduce the HIV-1 reservoir. Future research could focus on combining these scDbs with other therapeutic approaches to further enhance the clearance of HIV-1 infected cells.
Reference
1. Board, Nathan L., et al. “Bispecific antibodies promote natural killer cell-mediated elimination of HIV-1 reservoir cells.” Nature Immunology (2024): 1-9.
