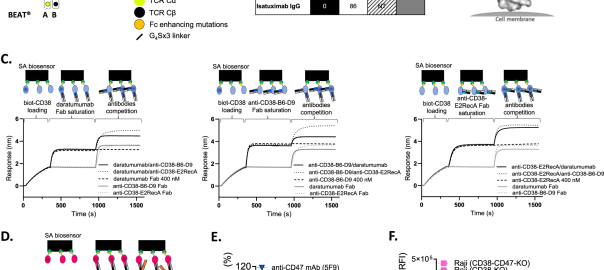
Antibody engineering is revolutionizing immuno-oncology, allowing for the synthesis of synthetic immunity by optimizing various components of antibody structure. Monoclonal antibodies (mAbs) and bispecific antibodies (BsAbs) with enhanced antibody effector functions haveRead More…
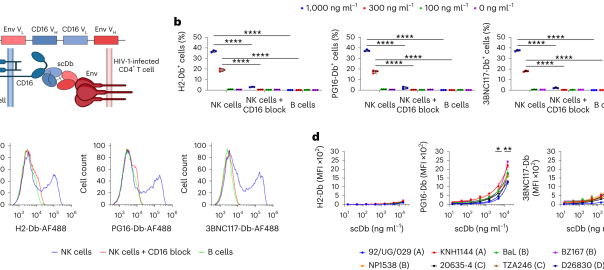
Advancing HIV-1 Therapy: Bispecific Antibodies to Enhance Latent Reservoir Clearance
Antiretroviral therapy can suppress HIV-1 replication, but the persistence of long-lived resting memory CD4+ T cells harboring latent pre-viral HIV-1 DNA prevents a cure. To date, clinical interventions based on Latency ReversalRead More…
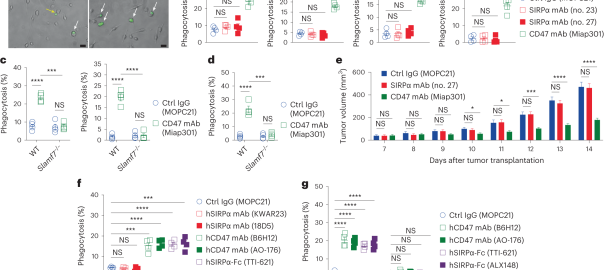
Unveiling a New Mechanism: How CD47 Shields Cancer Cells from Immune Clearance
Cancer cells often overexpress CD47, which induces the expression of the inhibitory receptor SIRPα on macrophages, thereby allowing them to evade phagocytosis and antitumor immunity. Pharmacological blockade of CD47 or SIRPα hasRead More…
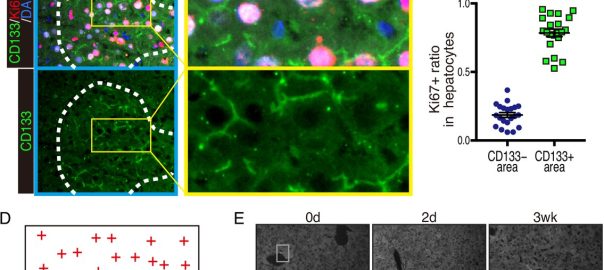
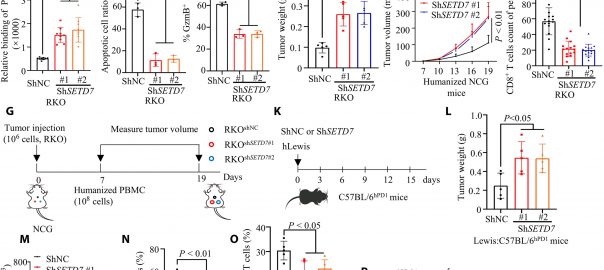
New Cancer Therapy Target May Disrupt Shared Resources Among Tumor Cells
CD133 widely recognized as a cancer stem cell marker associated with drug resistance and cancer relapse, has recently been the focus of a study published in the international journal eLife titled “IdentificationRead More…
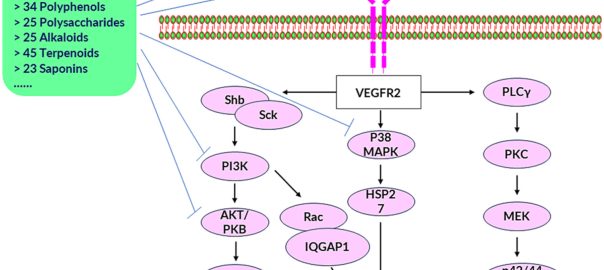
Natural Products Targeting Tumor Angiogenesis
Cancer is characterized by the abnormal development of cells that proliferate through uncontrolled cell division. It remains a significant global public health concern and is the second leading cause of death, trailingRead More…
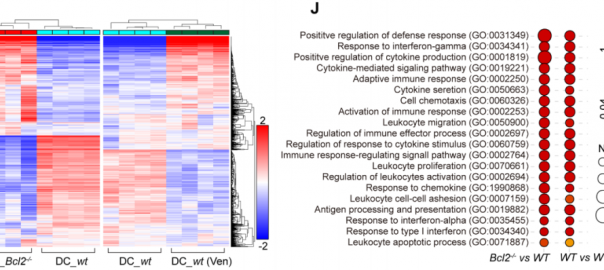
Scientists Confirm BCL-2 as an Immune Checkpoint for the First Time
Recently, a team of researchers from the Gustave Roussy Cancer Research Institute in France published a groundbreaking study in the journal “Cancer Discovery.” This study reveals, for the first time, that BCL-2Read More…
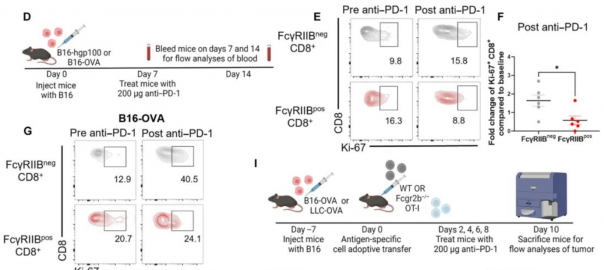
CD8-Positive T Cell Surface FcγRIIB Limits the Efficacy of PD-1 Inhibitors
Recently, a research team from Emory University in the United States published their latest research findings in “Science Translational Medicine,” revealing for the first time that the immune-inhibitory receptor FcγRIIB on theRead More…
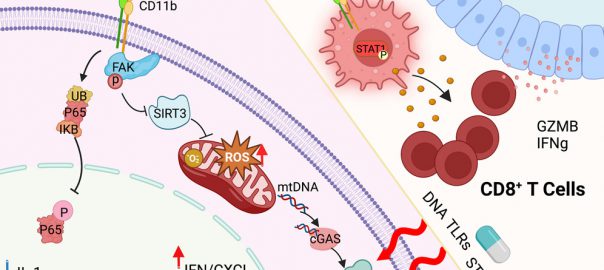
The Mechanism of CD11b Agonists in Exerting Anti-tumor Immune Response Has Been Unveiled
Using human tissue from a phase 1 clinical trial, researchers have demonstrated that treatment with GB1275, an oral small-molecule CD11b agonist, can activate the STING and STAT1 signaling pathways in human tumor-associatedRead More…
Researchers Have Found That PD-L1 Methylation Is a Key Mechanism for Immune Therapy Resistance
The PD-1/PD-L1 pathway, a critical immune checkpoint in the human body, is influenced by numerous regulatory factors, ranging from genetic mutations and variations to gut microbiota, as well as various factors insideRead More…
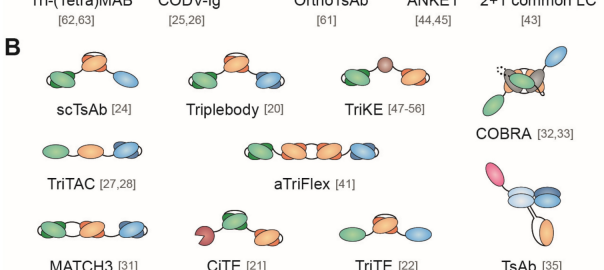
Multispecific Antibodies Enhance the Effectiveness of Cancer Immunotherapy
Immunotherapy is making significant advancements in oncology treatment. In 2021 alone, the FDA approved over a hundred monoclonal antibody drugs. Alongside monospecific antibodies, the development of multispecific antibodies is also in fullRead More…