Ten years ago, scientists announced the development of cancer immunotherapy called chimeric antigen receptor-T cell (CAR-T) therapy: genetically modifying T cells from patients to make them powerful anti-tumor effects, and then infusing these genetically modified T cells into the patient’s own body. Since then, CAR-T, one of several strategies collectively known as “adoptive T cell transfer”, has made headlines as a new tool for cellular immunotherapy. By far, they have been most successful in fighting so-called “liquid cancer”, such as leukemia and lymphoma.
Sarcomas and cancers are more resistant to these methods, in part because genetically modified T cells gradually lose their anti-tumor ability once they infiltrate the tumor. Immunologists refer to this cell fatigue as T cell “failure (exhaustion)” or “dysfunction”.
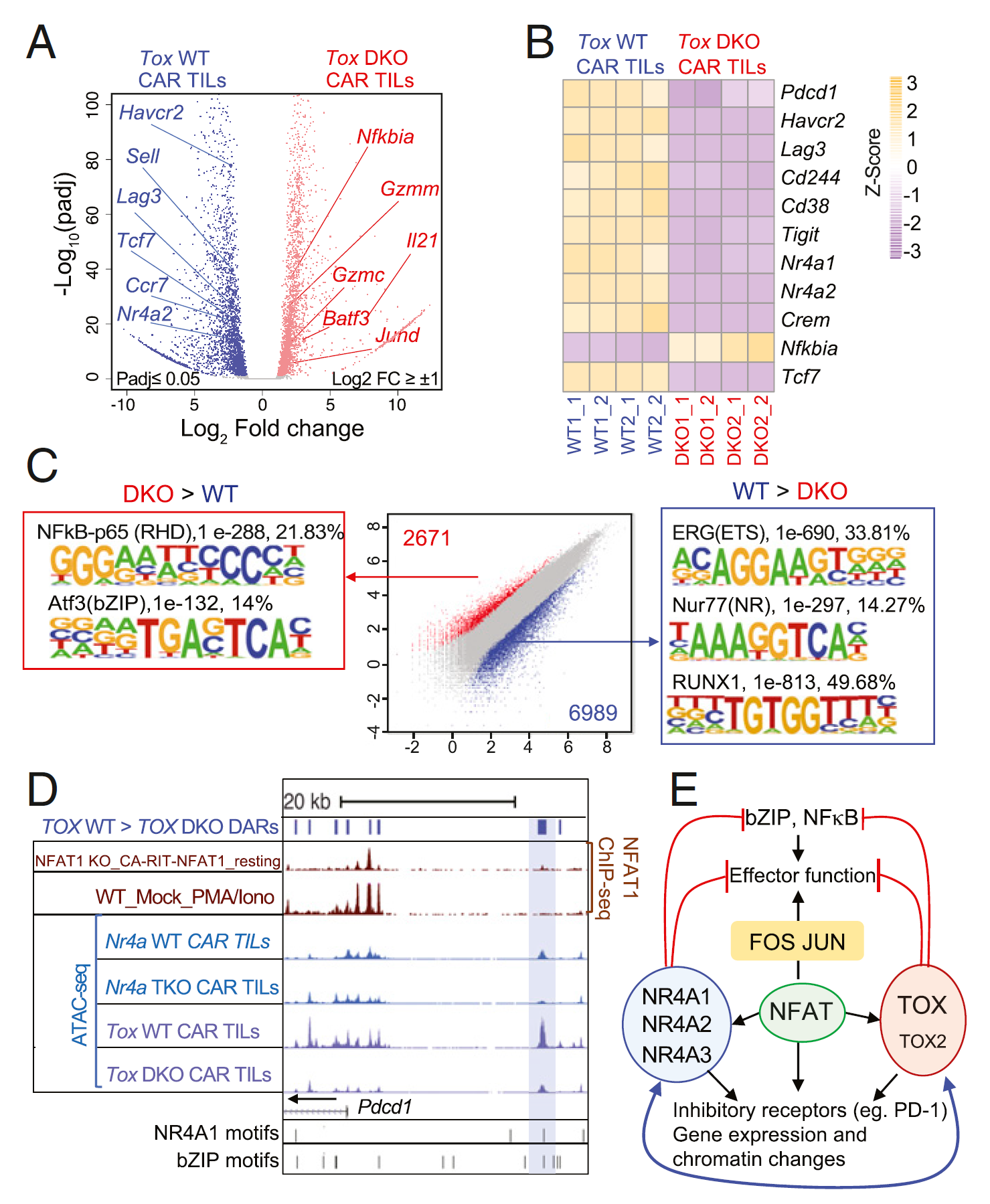
To understand why, Dr. Anjana Rao and Dr. Patrick Hogan of the Institute of Immunology in La Jolla have published a series of papers in the past few years, reporting that a transcription factor that regulates gene expression, called nuclear factor of activated T cells (NFAT) protein, turns on the “downstream” gene expression that weakens T cells’ anti-tumor response, leading to T cell failure. One of the downstream genes encodes the transcription factor NR4A, and the former graduate student Joyce Chen has found that the removal of NR4A protein from tumor-infiltrating CAR-T cells by genetic means can improve tumor rejection. However, the identity of the other participants who work in synergy with NFAT and NR4A in this approach is still unknown.
Now, in a new study, Rao Laboratory and Hogan Laboratory provide a more complete list of participants in an extensive gene expression network that establishes and maintains T cell failure. Using mouse models, the study found that genetic removal of two new transcription factors, TOX and TOX2, also improved the eradication of “solid” melanoma in the CAR-T method. It suggests that similar interventions targeting NR4A and TOX factors may extend the application of CAR-T-based immunotherapy to solid tumors. The results of the study, entitled “TOX and TOX2 cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion”, were published online in the journal PNAS on May 31, 2019.
They first compared gene expression profiles in normal and “failing” T cell samples, looking for factors that were up-regulated at the same time as NR4A as accomplices in T cell dysfunction. “We found that two DNA-binding proteins, called TOX and TOX2, are highly expressed with NR4A transcription factors,” said Dr. Hyungseok Seo, a postdoctoral researcher at Rao Laboratories and lead author of the paper. This finding suggests that factors such as NFAT or NR4A may control the expression of TOX. ”
They then recreated the CAR-T regimen in mice: first, melanoma cells were inoculated into mice to establish tumors, and then a week later, the mice were infused with one of two T cell populations: samples of “control” T cells from normal mice; and T cell samples from genetically modified T cells from mice that did not express TOX and TOX2.
It is worth noting that mice infused with TOX-deficient CAR-T cells showed stronger melanoma regression than mice receiving normal T-cell perfusion. In addition, mice treated with TOX-deficient CAR-T cells showed a significant increase in survival, suggesting that the lack of TOX factor antagonized T cell failure and allowed T cells to destroy tumor cells more effectively.
They found that the TOX factor was combined with NFAT and NR4A to promote the expression of an inhibitory receptor called PD-1. PD-1 appears on the surface of failing T cells and sends immunosuppressive signals.
PD-1 can be blocked by a number of monoclonal antibodies called immune checkpoint inhibitors, which resist immunosuppression and activate the innate anticancer immune response.
The convergence of TOX, NFAT, and NR4A on PD-1 makes sense both in molecule and immunology, which will enable people to combine cellular immunotherapy with antibody immunotherapy.
Seo said, “Currently, CAR-T cell therapy has shown amazing results for patients with ‘liquid tumors’ such as leukemia and lymphoma. However, because of T cell failure, they still cannot be used to treat patients with solid tumors. If we inhibit TOX or NR4A, by treating CAR-T cells with small molecules, this strategy may have a strong therapeutic effect on solid tumors such as melanoma.”
Reference
Seo, Hyungseok, et al. “TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion.” Proceedings of the National Academy of Sciences 116.25 (2019): 12410-12415.