Recently, it was announced that LineaRx (a part of Applied DNA Science), a company specializing in next-generation biotherapy, has achieved anti-CD19-CAR (chimeric antigen receptor) expression on human T cells through its proprietary non-viral, plasmid-free (NVPF) manufacturing platform. According to the company, this major event represents the first time an anti-CD19 antibody has been expressed on human T cells using a PCR-constructed linear DNA. The company believes the technology will be an important option for all gene therapies currently using virus delivery platforms, in particular the use of viral delivery synthetic genes to redirect patients’ T cells to tumor immunotherapy, known as CAR-T therapy.
Linearx’s unique PCR-based manufacturing technology creates linear DNA amplifiers that encode anti-CD19 connectors. And then the DNA constructs are delivered to human T cells in vitro by electroporation. Subsequent tests confirmed that these engineered T cells achieved anti-CD19 CAR expression. Although these preliminary data are encouraging, the overall expression level is low, and Linearx is continuing to use patent-pending high expression amplification (HEA) subtechnology and transfection methods other than electroporation to produce a high level of transfection. Further progress on this significant outcome will be reported at a later stage.
Dr. James A.Hayward, President and Chief Executive Officer of Applied DNA Science, said, “our goal is threefold: 1) to determine the value of linear DNA produced by PCR as a platform for the rapid design and production of therapeutic nucleic acid constructs; 2) demonstrate the safety and therapeutic value of improved NVPF processes that are different from current industry-wide virus-based production processes; and 3) work with future partners to promote the clinical application of NVPF-based anti-CD19 CAR-T cell therapy”.
Expression of GFP Gene fragment produced by PCR in Human T cells
This achievement is based on the first example of engineered human T cells produced, designed and manufactured by LineaRx PCR technology at the end of 2018. This example proves for the first time a linear DNA amplification containing a complete gene encoding green fluorescent protein (GFP). The GFP gene fragment produced by PCR is ingested and subsequently expressed in hundreds of human T cells by high-throughput electroporation, forming a green fluorescent cell population.
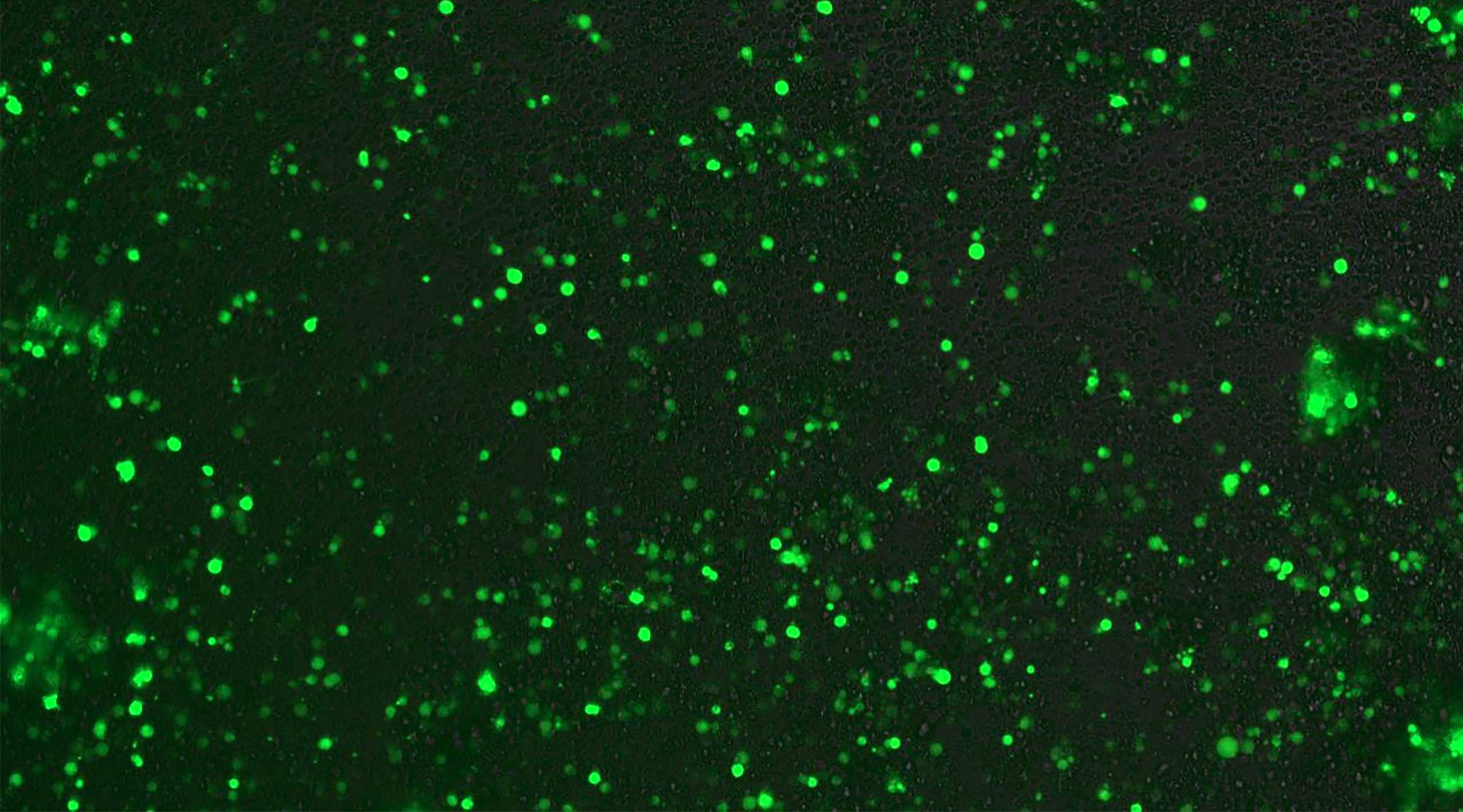 Expression of GFP Gene fragment produced by PCR in Human T cells
Expression of GFP Gene fragment produced by PCR in Human T cells
In October 2018, Linearx announced the signing of an exclusive North American licensing and research service agreement with Icell Gene Therapeutics for anti-CD19 CAR-T therapy. Combining Applied DNA’s expertise in large-scale PCR production and DNA chemical modification, the company will use its NVPF platform to develop and produce expression vectors for a variety of CAR-T therapies, including LinCART19, a non-viral, plasmid-free anti-CD19 CAR-T candidate.
As Linearrx discussed in December 2018, in a LinCART19-based CAR-T clinical trial in China, six months after a single low dose treatment, all 3 patients with acute lymphoblastic leukemia (ALL) achieved complete remission. Although these promising clinical results provide valuable evidence for the gene constructs used, CAR-T cells are transfected with viral vectors. LinCART19 will use linear DNA, and introduce T cells by electroporation, solubilization or otherwise. CD19 is a cell surface protein expressed in almost all B-cell malignant tumors, which is also the target of two approved CAR-T therapies.
Without the use of virus vectors or plasmids, Linearx’s NVPF manufacturing platform has many potential advantages over existing virus / plasmid-based CAR-T methods, including: 1) simplified manufacturing processes will reduce costs and delivery cycles; 2) reduce risk of permanent genetic integration / recombination into target cells (insertion mutagenesis); 3) potential remission of side effects of cytokine release storm; 4) the ultimate ability to target new antigens is sufficient to quickly affect patients; 5) avoid complex and cumbersome distributed supply chain and accelerate treatment.
Linearx believes that the NVPF method can produce important biotherapeutic drugs and is expected to redefine “bedside” therapy to provide patients with faster, more effective, more economical and safer gene therapy, turning personalized cancer therapy from a dream into a reality.
