Although complement was originally described as a powerful defense mechanism against invading pathogens, it can also be activated by tumor-targeting antibodies to kill tumor cells.
Antibody and Complement Activation
Immunoglobulin (Ig) induction of complement-dependent cytotoxicity (CDC) against tumor target cells requires the immobilization of C1q by at least two Ig-Fc fractions near the cell membrane. Attachment of C1q to the Fc portion of immunoglobulin occurs in the CH2 domain of the Ig molecule, and this binding is influenced by various factors, such as the type of antibody, the level of expression of the cell-surface antigen, the antibody-antigen binding capacity, the mobility of the cell-surface antigen, and many others.
Human IgG1, IgG3, and IgM can effectively immobilize C1q to their Fc portions, with the Fc portion of IgM more readily interacting with C1q. Due to its pentameric or hexameric structure, IgM is more likely to provide sufficient tight binding sites for C1q. On the other hand, the ability of IgG molecules to immobilize C1q is highly dependent on the level of antigen expression and varies among the four subclasses of IgG.
- IgG3 is more efficient at C1q binding than IgG1.
- lgG2 triggers complement recruitment only at high serum concentrations and high antigen expression levels.
- Human IgG4 completely lacks C1q binding capacity.
It has been shown that the strong complement activation ability of IgG3, relative to other IgGs is due to its higher molecular flexibility and longer hinge region (63 amino acids and 15 amino acids for IgG3 and IgG1 hinge regions, respectively). Based on these structural features, IgG3 molecules can span wider-spaced antigens than IgG1 molecules to immobilize C1q.
Since the central complement component, C3b, must be attached to the cell membrane, the entire complement cascade needs to be near the cell membrane. Antibody binding to different epitopes of the target antigen and the direction of binding also affect potency in terms of CDC. For example, upon binding to an antigenic epitope, an antibody is more effective at immobilizing C1q if the Fc end of the antibody is closer to the cell membrane and nearer to the Fc end of a neighboring antibody.
Strategies to Enhance Complement Activation by Therapeutic Antibodies
Various strategies are needed to improve complement activation by therapeutic antibodies. This is due to the lack of effective complement activation by some therapeutic antibodies and the fact that some tumor cells evade CDC through high expression of membrane-bound complement regulatory proteins (CRPs).
Regulation of Complement Regulatory Proteins
Most human cells express CRPs such as CD46, CD55, and CD59, which inhibit local complement activation and prevent uncontrolled CDC in different ways. To sensitize tumor cells to complement-mediated cell lysis triggered by tumor-targeting antibodies, CRPs (CD46, CD55, and CD59) can be blocked by neutralizing antibodies. Neutralizing antibodies should be used without the Fc portion that binds to C1q, ensuring that the complement only interacts with the tumor-targeting antibody to induce CDC, not with the neutralizing antibody.
Several monoclonal antibodies against CD46, CD55, and CD59 are available for use in in vitro blocking assays to assess the effects of CRP inhibition on CDC:
- Mouse Anti-Human CD46 Monoclonal Antibody (CTJS-69)
- Mouse Anti-Human CD55 Monoclonal Antibody (CTJS-530)
- Mouse Anti-Human CD59 Monoclonal Antibody (CTJS-540)
Antibody Engineering
Other approaches to enhance complement activation include generating bispecific antibodies that bind C1q with one arm and target cells with the other, and novel fusion proteins between the C3-binding domain of CR2 (human complement receptor 2) and the human IgG1-Fc portion. Both strategies have significantly improved complement-mediated cytotoxicity against target cells in vitro.
The initial complement component, C1q, binds to the CH2 structural domain of IgG1 antibodies. Idusogie et al. identified residues K326 and E333 as being primarily involved in C1q binding by substituting surface-exposed amino acids in the CH2 domain of IgG1. Moore et al. also analyzed 38 antibody variants and revealed that residues S267, H268, and S324 are essential for effective CDC. The CDC effect of antibodies can be enhanced by replacing important residues through antibody engineering.
We provide C1q-binding ELISA kits and C1q-binding assays for rapid assessment of changes in C1q binding capacity after antibody modification.
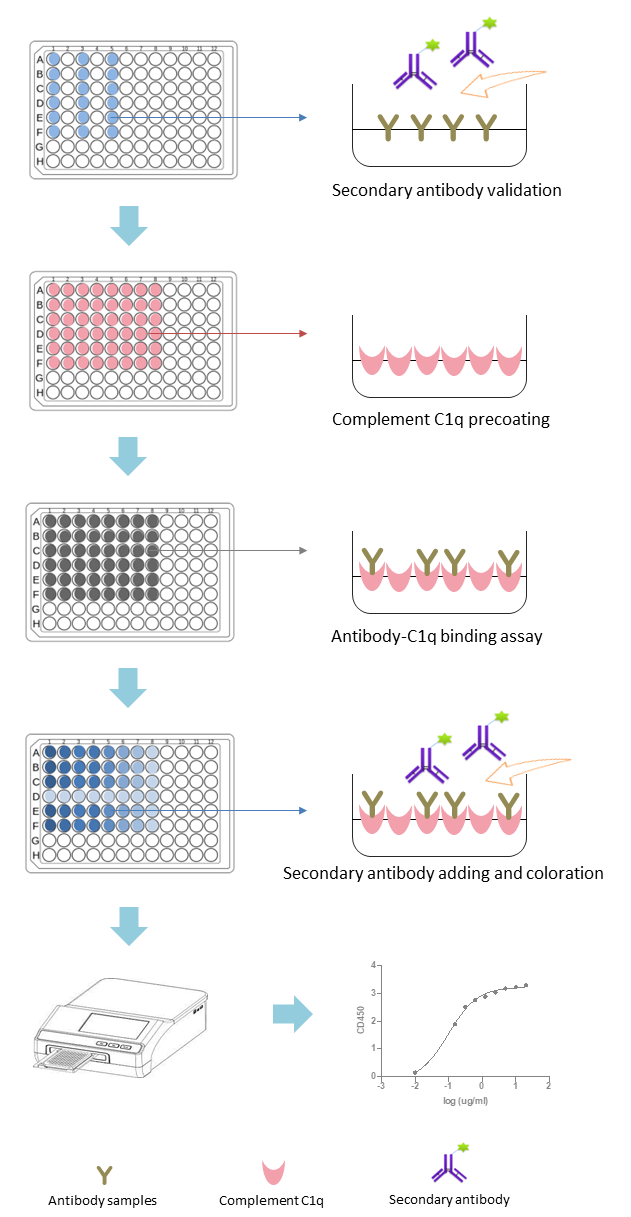
Fig.1 Workflow of Complement C1q-Binding Assays. (Creative Biolabs Original)
- Human Complement C1q ELISA Kit-CTK-002
- Human Complement C1q Antibody ELISA Kit
- Complement C1q-Binding Assays
Antibody Combination Strategy
There is a correlation between antigen expression levels and CDC effects triggered by antigen-specific antibodies. This can be achieved by a combination strategy using two non-competitive antibodies to increase the tight spacing on the target cells, which helps correctly localize the Fc portion that binds to C1q on the target cells. For example, the combination of two non-cross-blocking IgG1 antibodies (directed against the antifolate receptor, Her2/neu, or EGFR) strongly induces CDC compared to a single antibody.
