Oncolytic viruses have been proven to regulate the immune microenvironment, reduce suppressive immune phenotypes, and enhance anti-tumor immunity. Combining immune checkpoint inhibitors and oncolytic viruses is expected to overcome the resistance of immune checkpoint inhibitors.
Anti-tumor Immune Mechanism of Oncolytic Virus
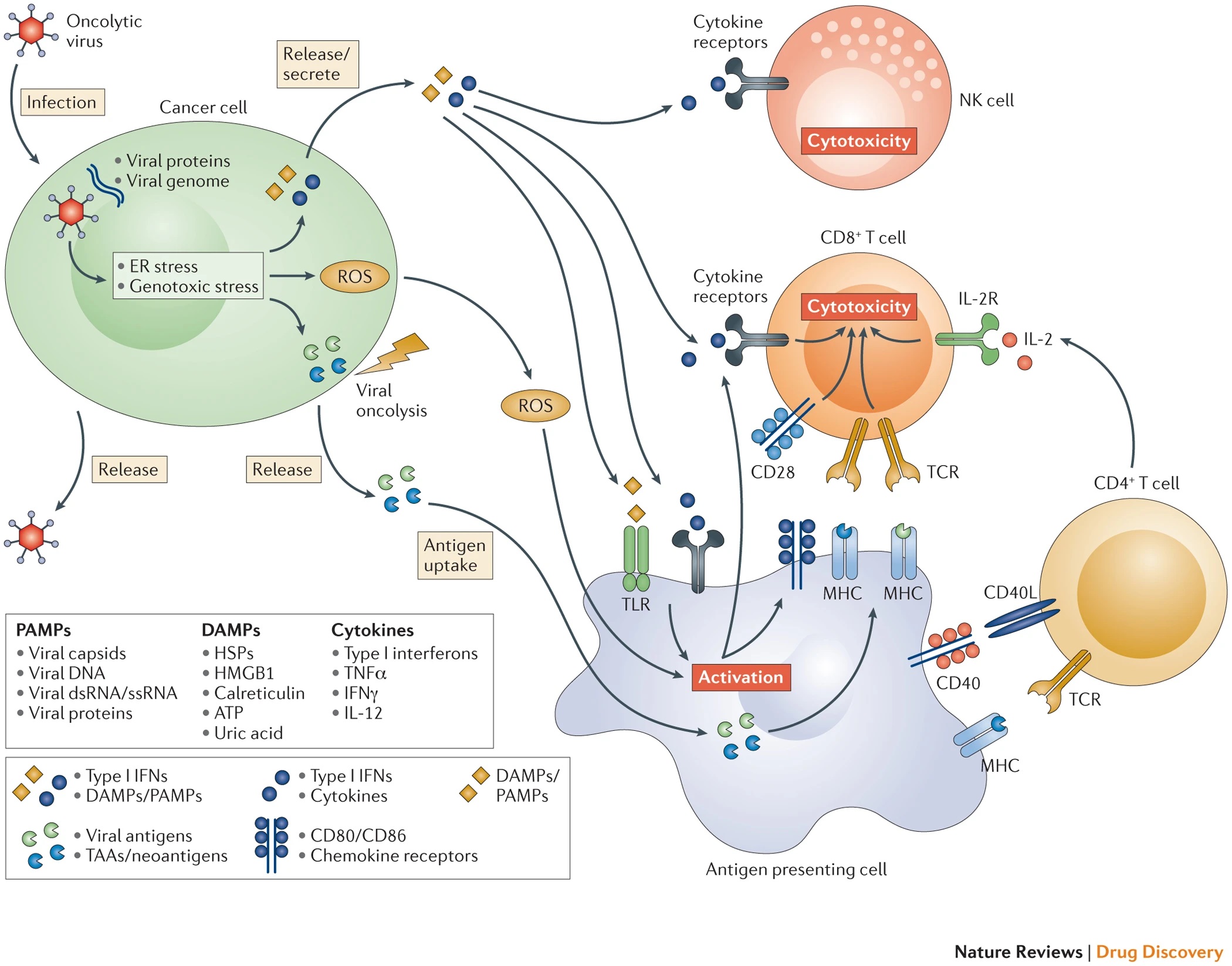
Oncolytic viruses can lyse tumor cells, release tumor-associated antigens and tumor-associated neoantigen (TAAs, TANs), which are captured by antigen-presenting cells such as DC, and activate T cell immunity.
They can also lead to the death of immunogenic cells (including necrosis, necroptosis, pyrolysis, autophagy, immunogenic apoptosis), and induce damage-associated molecular patterns (DAMPs). The viral components of oncolytic viruses induce pathogen-associated molecular patterns (PAMPs). DMAPs and PAMPs are signals of “danger” and “eat me” mediated by pattern recognition receptors on the surface of cells such as NK and DC. By driving more DC, NK, tumor-specific T cells to the tumor microenvironment, the immunity thereby is enhanced.
Tumor cells infected by the oncolytic virus can also induce the release of cytokines, driving more immune cells to the tumor microenvironment, which is transformed into a non-inhibitory state.
The Main Obstacles of Oncolytic Virus in Anti-tumor Immunity
- Producing neutralizing antibodies in the body can block viral infections.
- Anti-viral immune cell response can eliminate viruses.
- The physical barrier of the tumor can block oncolytic viruses.
- Genetically modified engineering can reduce the adaptability of the virus.
- Genetically modified products can also induce an immune response, leading to premature virus clearance.
Armed Strategies of Oncolytic Viruses
- Arming oncolytic viruses with tumor antigens
Oncolytic viruses lyse the tumor locally, releasing TAAs and TANs, which is similar to tumor vaccines. But sometimes it is not powerful enough to induce tumor-specific T cell responses. Thus the TAA encoding genes can be integrated into the oncolytic virus genome, allowing it to express a larger amount of TAAs (such as HER2, HPV E6, E7, MAGE-A3) to induce tumor-specific T cell immune responses.
The most commonly used cytokine is GM-CSF. The local release of GM-CSF by oncolytic virus can promote the maturation and migration of DC cells and enhance the immune response of T cells. IL-12 is a multifunctional cytokine that activates natural immunity and adaptive immunity. It is the main coordinator of Th1-type anti-tumor immunity and is also used to arm oncolytic viruses. Other cytokines, such as IL-2, IL-15 and IL-18, are also under investigation.
- Arming oncolytic virus with immune-activating ligands
At present, there are the most researches on armed oncolytic virus with CD40L. CD40 is a superfamily of tumor necrosis factor receptors. It is expressed on antigen-presenting cells( such as DC), and CD40L is expressed on activated CD4+ T cells, B cells, NK cells, and memory CD8+ T cells. Up to now, multiple CD40L oncolytic viruses are in clinical development, and the results show controlling tumor growth, inducing tumor cell apoptosis and T cell response, up-regulating Th1-type cell response and the proportion of effector T cells.
- Arming oncolytic viruses with chemokines
Chemokines are the largest subfamily of cytokines, which mediate immune cell migration and lymphatic tissue development. The chemokines under study include CCL5 (which can increase the local survival time of cell tumors), CLL19 (which can control tumor growth, enhance DC, CD4+ T cell migration to the tumor microenvironment), CCL20, and CCL21.
- Arming oncolytic viruses with Bi-specific T-cell Engager
Bi-specific T-cell Engager is the most commonly used molecular design scheme for bispecific antibodies, which recruits and activates T cells through CD3 antibodies, thereby killing target cells. There are currently some designs in the preclinical stage, such as EnAd-SA-EpCAM containing CD3 scFv and EpCAM scFv.
- Combination therapy of oncolytic virus and other tumor immunotherapy
Oncolytic viruses can be combined with immune checkpoint inhibitors (such as Ipilimumab and other therapeutic monoclonal antibodies targeting at immune checkpoints) for the treatment of a variety of cancers. At present, dozens of combined clinical applications are underway. In addition, oncolytic viruses can improve the inhibitory environment of the tumor microenvironment, and recruit immune cells.
Reference
1. Cook, Mary, and Aman Chauhan. “Clinical application of oncolytic viruses: a systematic review.” International journal of molecular sciences 21.20 (2020): 7505.
