Cytokine & Oncolytic Virotherapy Combination Therapy Development Service
Accelerate Your Cancer Immunotherapy Development!
How Creative Biolabs Can Assist Your Project?
Many researchers face challenges in developing effective cancer immunotherapies, including limited single-agent treatment efficacy, difficulties in achieving sustained anti-tumor responses, and coordinating multiple therapies. Our Oncolytic Virotherapy Development for Combination Therapy with Cytokines addresses these issues. By leveraging advanced vector engineering and comprehensive preclinical validation, we harness the synergistic effects of oncolytic viruses and cytokines to enhance the potency and durability of cancer immunotherapies.
Creative Biolabs provides a full range of services to support oncolytic virotherapy development projects from concept to preclinical validation. We offer customized solutions tailored to specific research needs, helping to accelerate the journey towards clinical success.
Workflow
| Required Starting Materials | Vector Design and Construction |
|---|---|
|
This includes, but is not limited to, providing the following information: Tumor cell lines or patient-derived xenografts (PDX models). Cytokine gene sequences of interest (IL-17, IL-12, IFN-γ, etc.). Specific therapeutic transgene(s) of interest (e.g., checkpoint inhibitors). |
Our expert team designs and constructs oncolytic viruses based on your specific requirements, including virus backbone selection, transgene insertion, and optimization of viral infectivity and replication. |
| In Vitro Validation | In Vivo Efficacy Studies |
| The engineered oncolytic viruses are rigorously tested in vitro to assess their tumor-selective cytotoxicity, transgene expression, and ability to enhance cytokine production. | Creative Biolabs conducts preclinical in vivo studies using relevant tumor models to evaluate the therapeutic potential of the oncolytic virus and its combination with cytokine therapy. This includes assessing tumor growth inhibition, survival rates, and immune response modulation. |
| Safety and Toxicology Studies | Data Analysis and Reporting |
| Comprehensive safety and toxicology studies are performed to evaluate the potential adverse effects of the oncolytic virus and the combination therapy, ensuring that the therapy is safe and well-tolerated. | Creative Biolabs provides detailed reports and data analysis, including statistical analysis, graphical representation of results, and interpretation of findings. |
| Final Deliverables | Estimated Timeframe |
|
Detailed study reports, including protocols, methodologies, and results. Raw and processed data from in vitro and in vivo experiments. Optimized oncolytic virus constructs products. |
The typical timeframe for this service ranges from 12 to 20 weeks, depending on the complexity of the oncolytic virus design, the specific requirements of the in vivo models, and the scope of the study. |
[Discover How We Can Help - Request a Consultation]
Case Study
The application of combination therapy integrating genetically modified oncolytic viruses and cytokines in commonly used in vivo rodent models and in vitro tumor cell line models, including those for prostate cancer, has resulted in a significant enhancement of anti-tumor activity. Findings from numerous published research studies provide valuable perspectives on its great potential for treating prostate cancer.
| Replication of Virus | Cytotoxicity | |
|---|---|---|
|
|
|
|
| Elisa | ||
|
|
|
|
| Flow Cytometry | ||
|
|
||
| Tumor Volume | Immunohistochemistry | |
|
|
|
|
Customer Reviews
-
"Enhanced Tumor-Specific Killing: Using Creative Biolabs' Oncolytic Virotherapy Development service in our research has significantly improved the tumor-specific killing efficacy of our oncolytic virus."
J**n[6 Months]
-
"Improved Preclinical Efficacy: Using Creative Biolabs' Oncolytic Virotherapy Development service in our research has significantly facilitated the demonstration of improved preclinical efficacy of our combination immunotherapy approach."
M**e[9 Months]
-
"Accelerated Development Timeline: Using Creative Biolabs' Oncolytic Virotherapy Development service in our research has significantly accelerated our development timeline for a novel oncolytic virus-based therapeutic."
D**d[12 Months]
[Experience the Creative Biolabs Advantage-Get a Quote Today]
What We Can Offer
As a leading provider of oncolytic virotherapy development services, Creative Biolabs offers biology experts:
- Customized Oncolytic Virus Design: We engineer oncolytic viruses tailored to your specific target tumor and therapeutic goals.
- Optimized Cytokine Expression: We fine-tune cytokine expression to maximize anti-tumor efficacy and minimize toxicity.
- Comprehensive Preclinical Validation: We conduct rigorous in vitro and in vivo studies to thoroughly evaluate the safety and efficacy of your oncolytic virus-cytokine combination therapy.
- Flexible Collaboration Models: We offer a range of collaboration models to suit your needs, from full-service support to specific project assistance.
- Specialist Advisory: Our group of seasoned scientists offers professional advice and assistance at every stage of the development journey.
Why Choose Us?
Creative Biolabs stands as your optimal collaborator for the advancement of oncolytic virotherapy in combination with cytokine-based treatment strategies.
- Expertise: Our team comprises experienced scientists with extensive knowledge in virology, immunology, and cancer biology.
- Advanced Technology: We utilize state-of-the-art technologies, including proprietary vector engineering platforms and high-throughput screening methods.
- Customized Solutions: We tailor our services to meet your specific research needs and project goals.
- Proven Track Record: Creative Biolabs has a history of successfully supporting clients in the development of novel cancer immunotherapies.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Introduction
Oncolytic viruses (OVs) represent a highly promising category of cancer treatments that selectively multiply within and eliminate tumor cells. In recent years, the advancement of OVs for cancer immunotherapy has attracted substantial interest. A multitude of preclinical and clinical investigations have illustrated their considerable potential.
Oncolytic Virotherapy Development for Combination Therapy with Cytokines
Combining oncolytic virotherapy with cytokines is a synergistic cancer immunotherapy approach. Oncolytic viruses modify the tumor microenvironment to promote anti-tumor immune responses, and cytokines, which are signaling proteins essential for immune modulation, further strengthen these responses. Pro-inflammatory cytokines such as IL-12 and GM-CSF can enhance T cell-mediated anti-tumor immunity, showcasing the great potential of this combined treatment.
Tab.1 List of IL-12 expressing oncolytic viruses and their efficacy in pre-clinical cancer models.2,3
| Oncolytic Virus | Strain | Cancer model | Administration | Efficacy |
|---|---|---|---|---|
| Adenovirus | RdB/IL-12/IL-18 | Melanoma | I.T. | 95% and 99% tumor growth inhibition |
| YKL-IL12/B7 | Melanoma | I.T. | Tumor growth is suppressed | |
| Ad-AB7/IL12/GMCSF | Melanoma | I.T. | Inhibited intracranial tumor growth and extended survival | |
| Ad-TD-IL-12, Ad-TD-nslL-12 | C2prostate adenocarcinoma | I.T. | Increased NK and CTL cytolytic activities; Increased survival | |
| HSV | G47Δ-mlL12 | Glioblastoma | I.T. | Promoted lL-12 expression, stimulated lFN-γ production |
| T-mflL12 | Neuroblastoma | I.V. | Prolonged survival | |
| NV1042 | Squamous Cell Carcinoma | I.T./I.V. | Reduced tumor volume and improved survival | |
| M002 |
Brain metastasized breast cancer |
I.T. | M002 significantly prolonged survival | |
| VSV | VSV-IL12 | squamous cell Carcinoma | I.T. | Significant reduction in tumor volume, and prolonged survival |
| NDV | rClone30IL-12 | Hepatocarcinoma | I.T. | Reduced tumor volume and improved percentage of survival |
Creative Biolabs is dedicated to providing cutting-edge solutions for oncolytic virotherapy development for combination therapy with cytokines. Our expertise and technology can help you accelerate your research and achieve your goals in cancer immunotherapy. If you have any need, please feel free to contact us!
References
- Yan, Wenyi, et al. "Oncolytic Vaccinia Virus Armed with GM-CSF and IL-7 Enhances Antitumor Immunity in Pancreatic Cancer." Biomedicines 13.4 (2025): 882. DOI: 10.3390/biomedicines13040882.
- Nguyen, Hong-My, Kirsten Guz-Montgomery, and Dipongkor Saha. "Oncolytic virus encoding a master pro-inflammatory cytokine interleukin 12 in cancer immunotherapy." Cells 9.2 (2020): 400. DOI: 10.3390/cells9020400.
- Distributed under Open Access license CC BY 4.0, Some of the pictures were edited and reformatted.

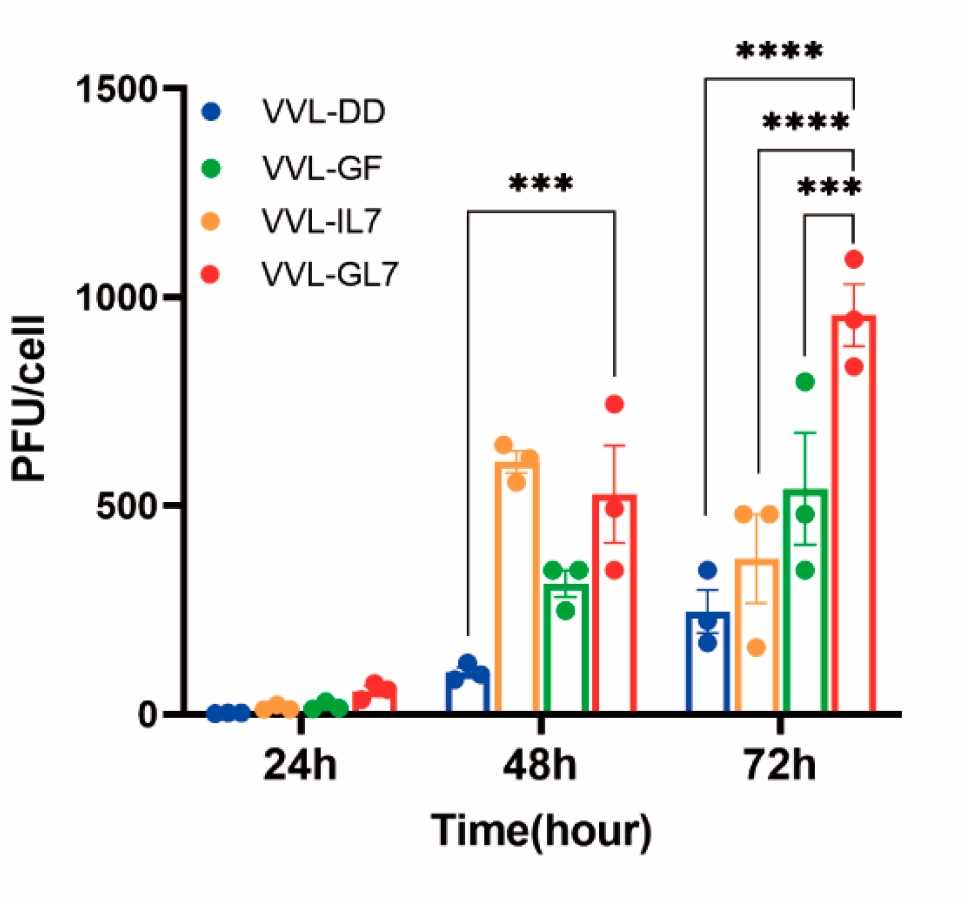 Fig.1 Titration of TCID50 reveals the replication ability of the virus.1,3
Fig.1 Titration of TCID50 reveals the replication ability of the virus.1,3
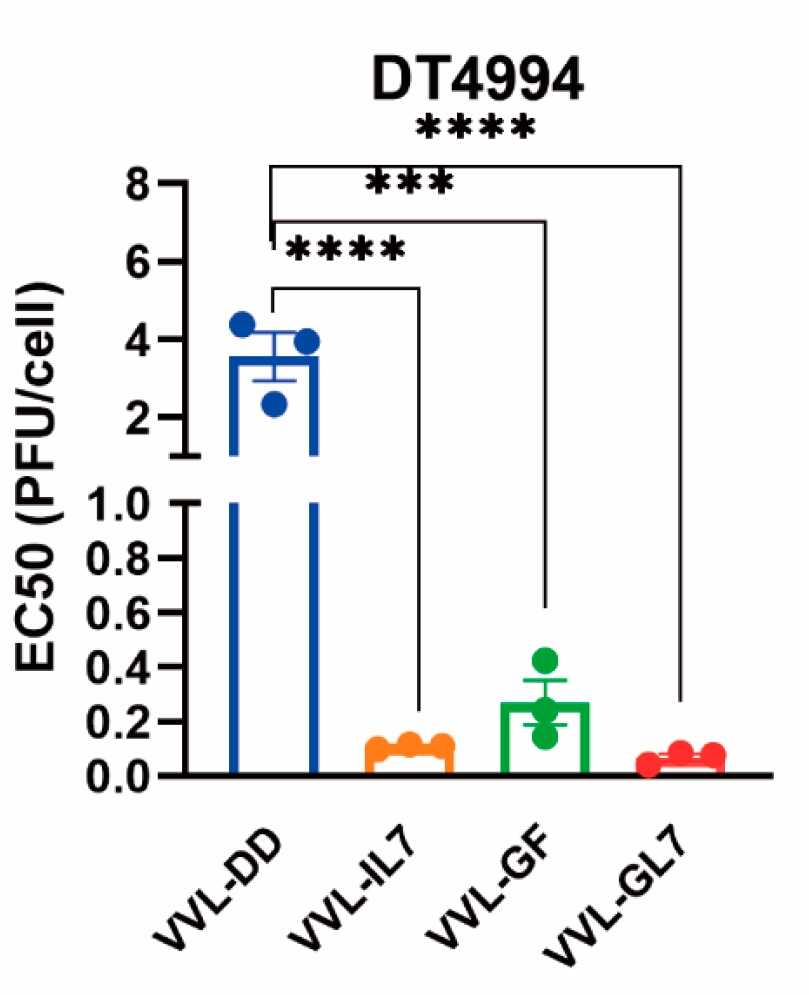 Fig.2 The EC50 of oncolytic viruses is used to represent viral cytotoxicity.1,3
Fig.2 The EC50 of oncolytic viruses is used to represent viral cytotoxicity.1,3
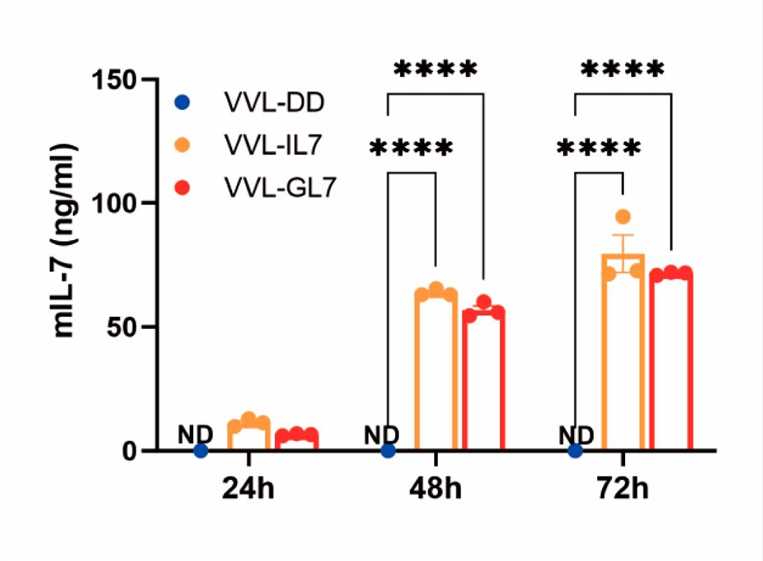 Fig.3 ELISA is used to quantify IL-7 secreted by the cells.1,3
Fig.3 ELISA is used to quantify IL-7 secreted by the cells.1,3
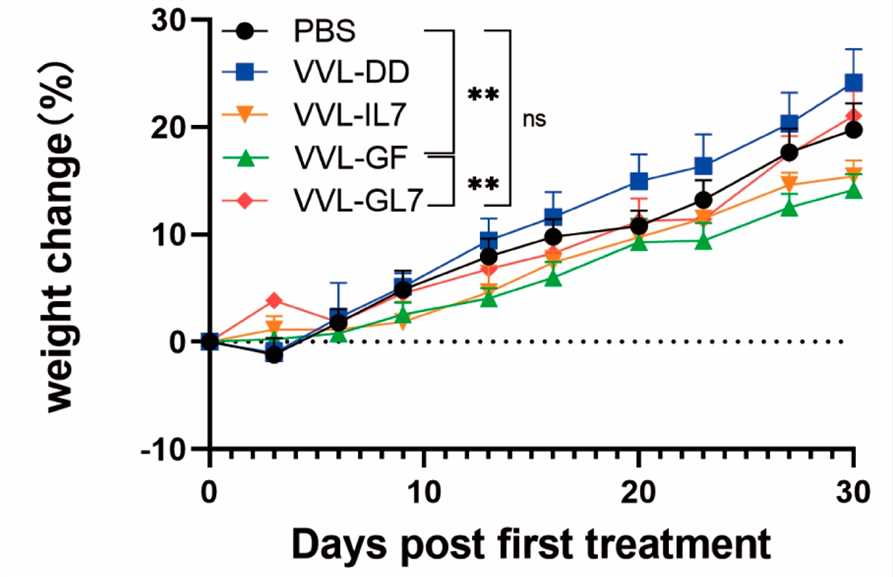 Fig.4 The combination of oncolytic virus and cytokine therapy could stabilize the body weight of mice.1,3
Fig.4 The combination of oncolytic virus and cytokine therapy could stabilize the body weight of mice.1,3
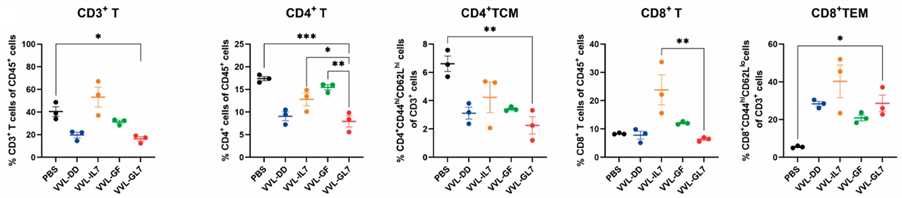 Fig.5 The changes of immune cells are detected by flow cytometry.1
Fig.5 The changes of immune cells are detected by flow cytometry.1
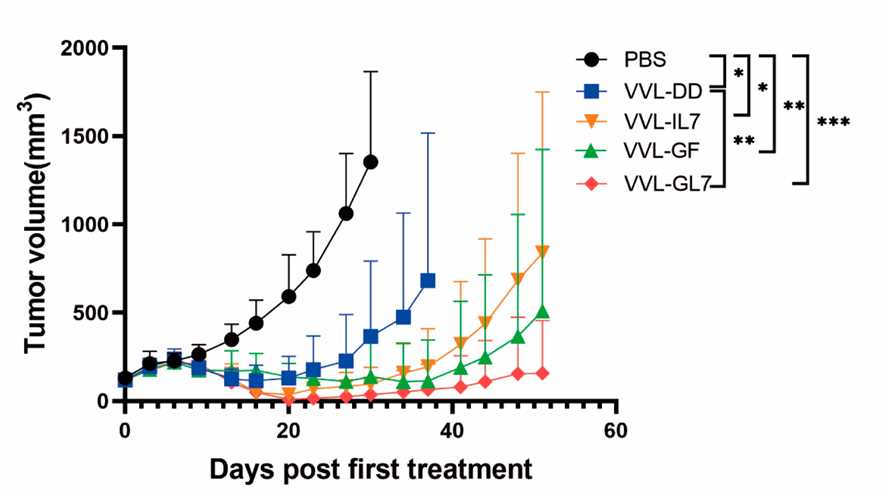 Fig.6 Oncolytic virus combines with cytokine therapy slowed tumor growth.1,3
Fig.6 Oncolytic virus combines with cytokine therapy slowed tumor growth.1,3
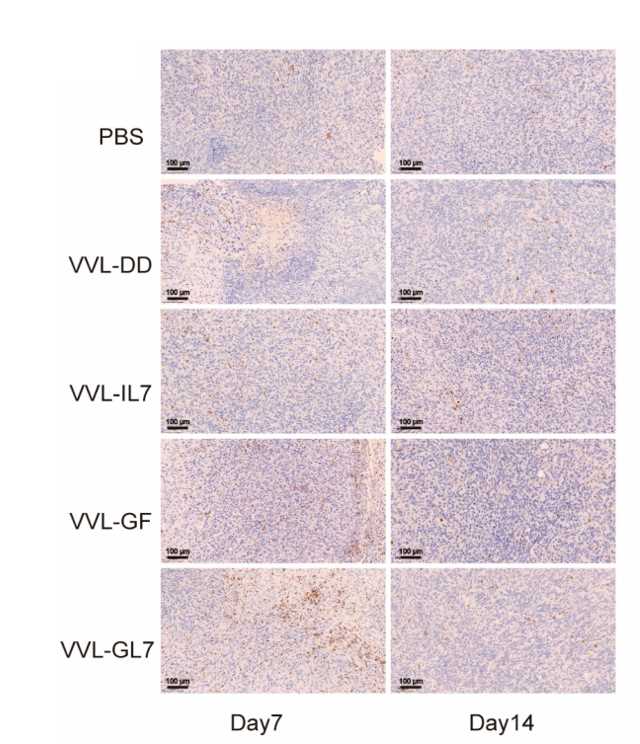 Fig.7 Tumor-infiltrating lymphocytes were quantified by immunohistochemistry.1,3
Fig.7 Tumor-infiltrating lymphocytes were quantified by immunohistochemistry.1,3