The development concept of single domain antibody-drug conjugates (sdAb-drug conjugates) is similar to that of antibody-drug conjugates (ADCs). That is, the antibody in the ADC is replaced with a single domain antibody with a higher affinity. Compared with ADCs, sdAb-drug conjugates have the advantages of a fast clearance rate, strong stability, and strong tumor penetration ability.
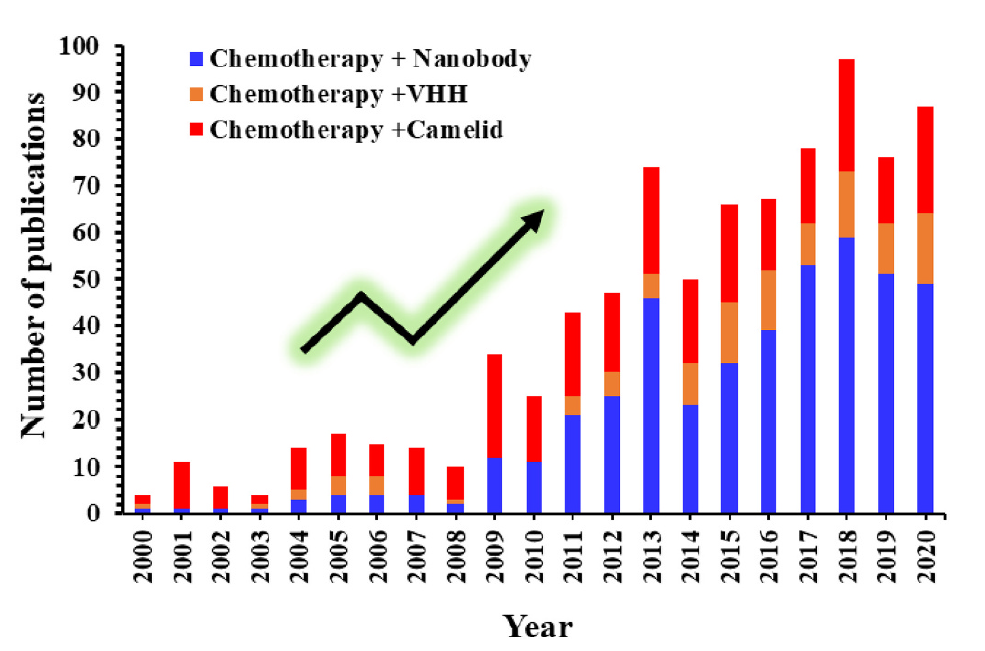
Fig. 1 Number of publications related to single domain antibodies in chemotherapy from 2000 to 2020 (Panikar SS, 2021)
Potential advantages of sdAb-drug conjugates compared to mAbs in drug development
Tissue penetration: The larger size and presence of the Fc region of mAbs can improve pharmacokinetic properties, but also affect their tissue penetration ability. Smaller volumes of sdAb-drug conjugates enable higher tissue penetration and enhanced cell killing in vivo.
Antigen recognition ability: Among all complementarity determining regions, CDR3 accounts for 60–80% of antigen recognition specificity. The CDR3 loop is long and extended, which endows VHH with better antigen recognition specificity and affinity, which can enhance the recognition of hidden tumor antigen epitopes.
Strong stability: Compared with mAbs, sdAbs have significantly higher thermal stability, Tm values, and reversible thermal denaturation. In addition, sdAbs are resistant to the denaturing effects of proteases, extreme pH, and hydrophobic agents.
Tandem into a multispecific or multivalent configuration: Compared with mAbs, sdAbs are small in size, which is conducive to tandem with a variety of antibody domains with different functions and can be used for the delivery of multifunctional drugs. It can also avoid rapid renal clearance and increase the drug ratio while enhancing affinity.
Taking these characteristics together, compared with mAbs or their fragment counterparts, sdAbs are ideal candidates for the development of highly stable and specific drug-conjugated carriers.
Key factors to consider in the development of sdAb-drug conjugates
In order to improve the efficiency of sdAb-drug conjugates, several factors can be considered—enhancing the specificity and affinity of sdAb-drug conjugates, the selection of conjugated drugs, site-specific attachment strategies, drug sites, and the ratio of drugs to sdAb-drug conjugates.
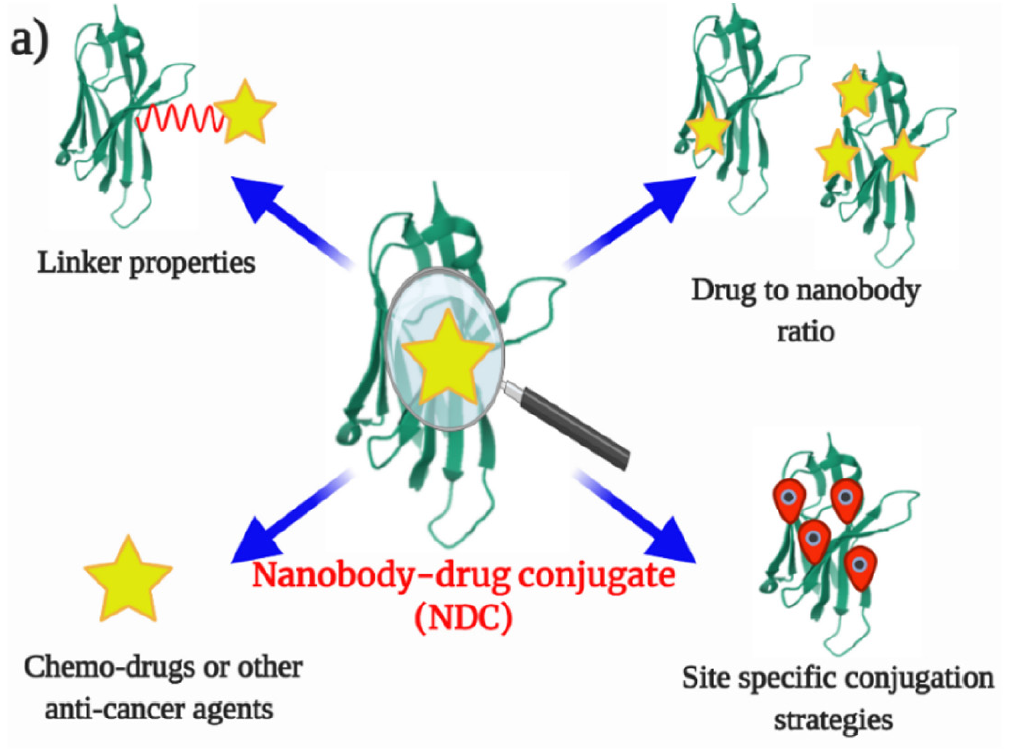
Fig. 2 Factors to consider when developing sdAb-drug Conjugates (Panikar SS, 2021)
Drug type: The drug conjugates used to prepare sdAb-drug conjugates are mainly divided into DNA damage agents and microtubule inhibitors.
Linker: Linker molecular design is a key factor affecting pharmacokinetics and pharmacodynamics. In order to maximize the efficiency of sdAb-drug conjugates, the ideal linker should meet the following conditions:
- High stability in human plasma
- It can break itself under specific tumor environments
- Use a hydrophilic linker to reduce the aggregation of sdAb-drug conjugates.
Ratio of drug to antibody: Although theoretically sdAbs do not show drug-loading advantages over mAbs, the effective accumulation and stability of sdAb-drug conjugates at the tumor site are superior to those of ADCs. The robust properties of sdAb-drug conjugates (high antigen specificity, stability, solubility, and lower immunogenicity) will allow them to enter target cells more efficiently than mAbs.
Site-specific conjugation strategy: Compared with ADCs, the simple chemical conjugation strategy of sdAb-drug conjugates and drugs has wider applicability, even surpassing ADCs in terms of low production cost and long-term stability. For sdAb-drug conjugates, lysine, cysteine, aspartic acid, and glutamic acid are usually selected as chemical conjugation sites to achieve a higher ratio of drug to sdAbs. However, this may also result in a heterogeneous mixture of sdAb-drug conjugates, affecting overall potency. In recent years, advances in protein chemistry have enabled the use of new tools to achieve homogeneous conjugation of drugs to sdAbs, including lysine amide coupling, insertion of cysteine residues, insertion of unnatural amino acids, and enzymatic conjugation, among others.
In clinical practice, although therapeutic antibody drugs have strong targeting, their therapeutic effect on solid tumors is limited due to their large molecular weight. Although small-molecule chemical drugs have a high killing effect on cancer cells, their selectivity is poor, and they often accidentally injure normal cells, causing serious side effects. As sdAb-drug conjugates are receiving more and more attention in diagnosis and therapeutic applications, chemotherapy drugs based on sdAb-drug conjugates are expected to achieve better efficacy in tumor treatment.
Reference
Panikar SS, et al. Nanobodies as efficient drug-carriers: Progress and trends in chemotherapy. J Control Release. 2021 Jun 10;334:389-412. doi: 10.1016/j.jconrel.2021.05.004. Epub 2021 May 6. PMID: 33964364.
