Single domain antibodies (sdAbs) are the smallest units that retain antigen-binding sites. They have the advantages of high antigen affinity, high specificity, high solubility, high stability, high clearance, low immunogenicity, and can be produced by microorganisms. Currently, two single-domain antibody therapeutic drugs have been approved for marketing. But in the field of tumor imaging, single-domain antibodies are still in the preclinical and clinical development stages.
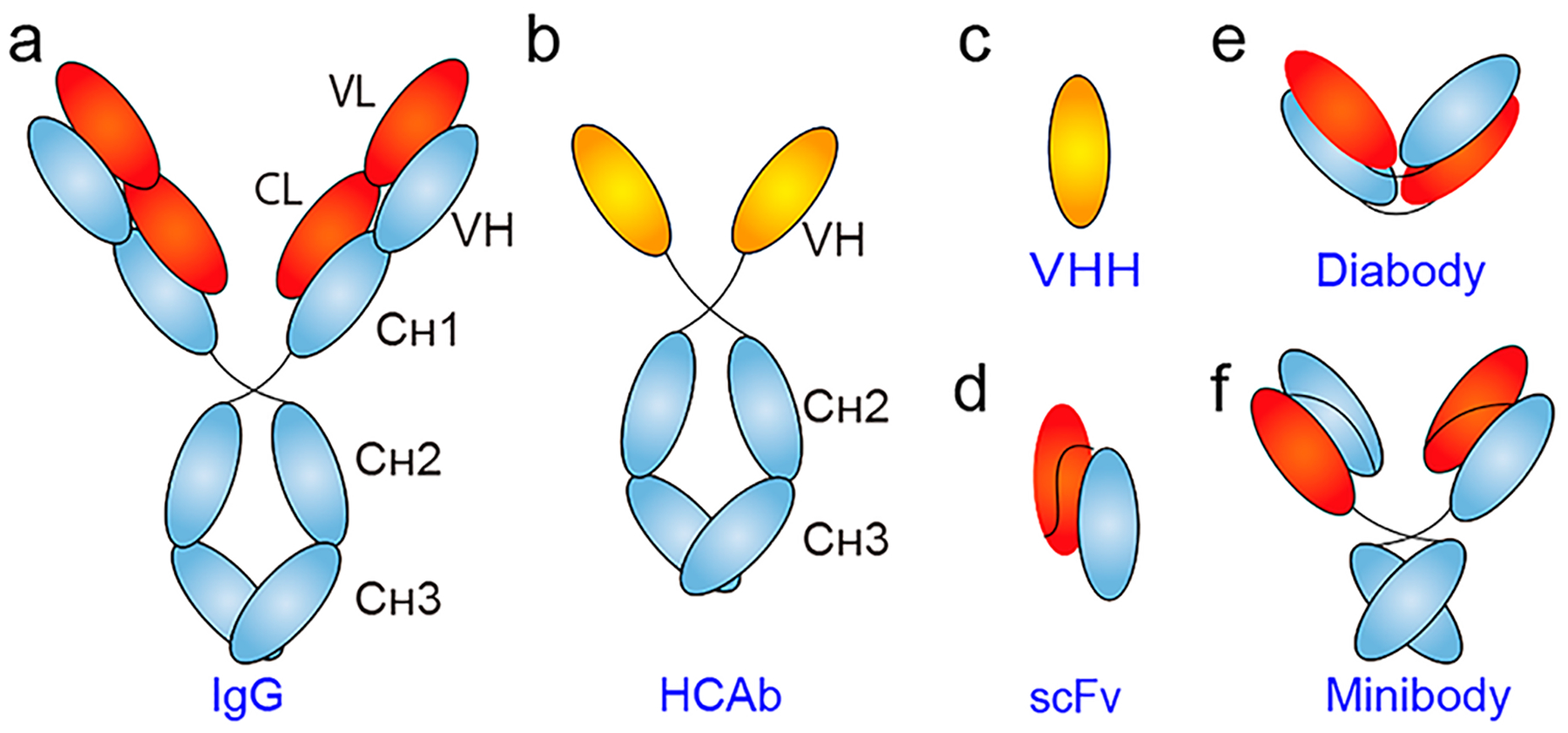
Fig. 1 Schematic of representative antibody and antibody fragments. (Wei, 2020)
- Introduction to EGFR targets
Human epidermal growth factor receptor (EGFR), also known as ERBB1 or HER1, is a transmembrane receptor tyrosine kinase (RTK) belonging to the ErbB receptor tyrosine kinase family. EGFR gene amplification, duplication, mutation, deletion, or whole-code mutation may lead to increased expression levels or functional activation of EGFR, which is associated with a poor prognosis of tumors. Multiple studies have shown that overexpression of EGFR has been detected in colorectal cancer, lung cancer, breast cancer, ovarian cancer, cervical cancer, bladder cancer, esophageal cancer, gastric cancer, head and neck cancer, and endometrial cancer. EGFR is a relatively mature target in the field of tumor treatment. It consists of three different domains, including an extracellular domain, a transmembrane domain, and an intracellular domain. Currently, a number of tyrosine kinase inhibitors (TKIs) targeting the intracellular domain of EGFR have been approved for marketing; in addition, a number of monoclonal antibodies targeting the extracellular domain of EGFR have been approved for marketing. In terms of tumor diagnosis, targeted drugs, such as specifically labeled EGFR antibodies, can detect cells overexpressing EGFR.
- EFGR single domain antibodies under research
According to literature reports, researchers immunized camels with EGFR-overexpressing cells and obtained EGFR-specific single domain antibodies. Similar to monoclonal antibodies, single domain antibodies mainly target the extracellular domain (ECD) of EGFR. A number of EGFR single domain antibodies have been developed for the diagnosis and treatment of EGFR overexpression tumors, including αEGFR-αEGFR-Alb, 8B6, 7D12, 7C12, EG2, 9G8, B39, EGa1, D10, etc. Generally speaking, most monovalent single domain antibodies are used for tumor imaging, and the commonly used expression system is E. coli. To perform tumor imaging, single domain antibodies also need to be linked to imaging labels.
- EFGR single domain antibody labeling strategy
3.1 Radioactive metal element-labeled single domain antibodies
Taking 99mTc as an example, some studies have reported the feasibility of EG2 as a molecular probe for detecting EGFR overexpressing tumor cells. The study labeled the EGFR single domain antibody EG2 with a tricarbonyl kit, and then tested the specificity of 99mTc-EG2 on A431 (EGFR overexpressing cells) and OCM-1 (EGFR low expressing cells) cell lysates. Its binding affinity is approximately 43.53 nmol/L. SPECT results showed that 99mTc-EG2 had a short half-life and a fast blood clearance rate, and EGFR overexpressing cells were observed one hour after injection. Another study reported detecting a reduction in tumor burden during EGFR TKI treatment using 99mTc-7C12 as a noninvasive tracer. 99mTc-D10 has also reported application potential for imaging small tumors or early-stage tumors, where tumors smaller than 100 mm in size can be visualized by SPECT imaging. This also means that 99mTc-D10 has the ability to evaluate EGFR expression levels during disease progression and may be a good tracer for non-invasive detection of tumors.
68Ga and 89Zr are two other commonly used radiolabeled elements. Some researchers have prepared conjugates 68Ga-7D12 and 89Zr-7D12 based on the EGFR single domain antibody 7D12. The results showed that biodistribution and PET imaging experiments of 68Ga-7D12 and 89Zr-7D12 in mice with A431 transplants showed similar tumor uptake. Compared with radiolabeled monoclonal antibodies, 68Ga-7D12 showed stronger image contrast at early time points. The above results highlight the potential of 68Ga-7D12 for evaluating EGFR overexpressing tumors.
3.2 Near-infrared fluorescent dye-labeled single domain antibodies
The use of near-infrared fluorescence technology for in situ imaging of tumor surgery has high application potential, allowing doctors to see the surgical steps instantly. Some studies have used the near-infrared fluorescent dye IRDye800CW to label 7D12 (EGFR single domain antibody) and EGFR monoclonal antibodies and compared the tumor imaging potential of the two. The results showed that 7D12-IR imaging of tumor cells was observed 30 minutes after injection, while the EGFR monoclonal antibody produced no signal. Tumor uptake was observed 2 hours after injection of 7D12-IR, and EGFR mAb accumulated in tumor cells after 24 hours. There are also studies comparing 7D12 labeled with different substances. The results show that 7D12 labeled with near-infrared fluorescent dyes has a higher tumor uptake efficiency than 7D12 labeled with radioactive elements, reflecting the excellent fast optical imaging performance of 7D12-IR.
3.3 Crystal nanoparticle-labeled single domain antibodies
Cobalt tetroxide (Co3O4) nanoparticles are crystalline nanoparticles based on their morphology and peroxidase-like (POD) activity. Some researchers have chosen to combine the EGFR single domain antibody B39 with Co3O4 nanopolyhedrons to detect EGFR overexpressing cells. The coupling strategy of B39 and crystal nanoparticles is to couple the nanoparticles to the C-terminus of the single-domain antibody B39, leaving the N-terminus containing the EGFR epitope completely free for detection of EGFR overexpressing cells. In addition, the researchers found that the conjugate had a low tendency to aggregate.
3.4 Quantum dot-labeled single domain antibodies
Quantum dots (QD) are a kind of semiconductor nanocrystal material. When excited by ultraviolet light, the quantum dots are in a high-energy state and release light of another wavelength. The wavelength depends on the size of the nanoparticles. QD-EG2 is the first imaging agent developed for tumor diagnosis. It is formed by coupling the EGFR single domain antibody EG2 and quantum dots. Other similar EGFR cell imaging reagents are QD-7D12, QD-EgA1 and QD-EgB. Quantum dots carrying the anti-cancer drug aminoflavonoids and coated with 7D12 have therapeutic, diagnostic and therapeutic properties. The single-domain antibody 7D12 enhances tumor uptake and delivers the drug to deep areas of the tumor.
3.5 Contrast agent labeled single domain antibodies
Contrast media is also an imaging marker. The iRGD (CRGDKGPDC) peptide is a type of CPP that can be localized on the cell membrane and enter the cell interior through the integrin receptor αVβ3. In order to improve the specificity of iRGD, the researchers designed the conjugate B39-iRGD of the EGFR single domain antibody and iRGD. B39-iRGD shows potential for tumor treatment and diagnostic applications. Compared with single domain antibodies alone, B39-iRGD combined with radiotherapy can enter deep areas of tumor cells and has stronger anti-tumor activity. In terms of imaging applications, the anti-EGFR-iRGD-DTPA-Gd obtained by coupling the single-domain antibody-iRGD with the contrast agent Magnevist can be combined with magnetic resonance imaging (MRI) technology to detect gastric cancer. (Learn more about our sdAb conjugation services)
References
Piramoon M, Khodadust F, Hosseinimehr SJ. Radiolabeled nanobodies for tumor targeting: From bioengineering to imaging and therapy. Biochim Biophys Acta Rev Cancer. 2021 Apr;1875(2):188529. doi: 10.1016/j.bbcan.2021.188529.
Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: Concept, Design, and Applications. Chem Rev. 2020 Apr 22;120(8):3787-3851. doi: 10.1021/acs.chemrev.9b00738.
Tang H, Gao Y, Han J. Application Progress of the Single Domain Antibody in Medicine. Int J Mol Sci. 2023 Feb 20;24(4):4176. doi: 10.3390/ijms24044176.
Yang E, Liu Q, Huang G, Liu J, Wei W. Engineering nanobodies for next-generation molecular imaging. Drug Discov Today. 2022 Jun;27(6):1622-1638. doi: 10.1016/j.drudis.2022.03.013.
Brilhante-da-Silva N, de Oliveira Sousa RM, Arruda A, Dos Santos EL, Marinho ACM, Stabeli RG, Fernandes CFC, Pereira SDS. Camelid Single-Domain Antibodies for the Development of Potent Diagnosis Platforms. Mol Diagn Ther. 2021 Jul;25(4):439-456. doi: 10.1007/s40291-021-00533-7.
Sharifi J, Khirehgesh MR, Safari F, Akbari B. EGFR and anti-EGFR nanobodies: review and update. J Drug Target. 2021 Apr;29(4):387-402. doi: 10.1080/1061186X.2020.1853756.
