Expressed Protein Ligation for Glycoprotein Synthesis
Expressed protein ligation (EPL) is a method for generating recombinant thioesters that is based on the phenomenon of protein splicing. The application of EPL for glycoprotein synthesis is advantageous because it enables the fusion of synthetic glycopeptides with recombinant protein fragments. As a leading specialist in glycodesign and glycoprotein synthesis, Creative Biolabs has gained significant knowledge in employing EPL for glycoprotein synthesis. We are more than happy to share our experience and help our customers in glycoprotein synthesis.
Background of Glycoprotein Synthesis
The glycoprotein saccharides contribute physiochemical properties, influencing protein conformation, or increasing stability against the proteolytic activity. With their unique structural diversity and complexity, carbohydrates attached to proteins are involved in numerous cell-surface binding events, such as cell growth and differentiation, cell proliferation, cell adhesion, fertilization, and immune responses. Structurally defined glycoproteins, which contain information about the glycan structure and glycosylation sites, are valuable for functional biological studies. To comprehensively understand how glycosylation regulates protein function, homogeneous glycoproteins are greatly needed.
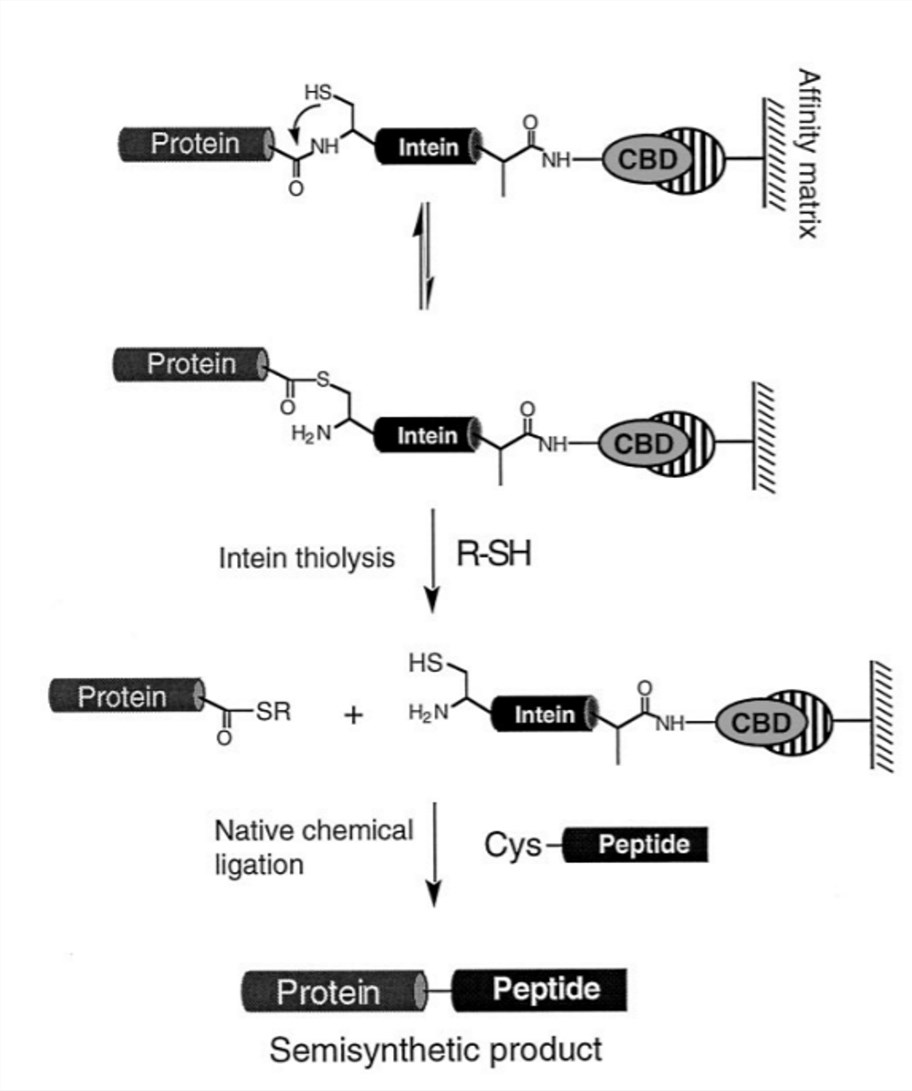 Fig.1 Recombinant protein α-thioesters and expressed protein ligation. (Muir, 2003)
Fig.1 Recombinant protein α-thioesters and expressed protein ligation. (Muir, 2003)
Introduction of EPL
Scientists have introduced an elegant method termed native chemical ligation (NCL) for the synthesis of peptides by condensation of their unprotected segments. This method has proven to be useful for the synthesis of smaller proteins up to 120 amino acids in length and larger proteins can be obtained by multistep NCL. EPL is an extension of this NCL strategy, using recombinant thioesters and/or αCys-peptides. A recombinant Cα-thioester reacts with an expressed or chemically synthesized peptide/protein possessing an N-terminal Cys to form a native peptide bond. Combining the advantages of molecular engineering and chemical peptide synthesis, this ligation method allows the site-specific introduction of unnatural amino acids and chemical tags into large proteins. EPL has been extensively used for the preparation of homogenous glycoprotein samples. The use of EPL was first reported for the ligation of a 392-residue intein-generated α-thioester and N-Cys dipeptide functionalized with a single N-acetylgluocasmine residue. EPL was also used for the semi-synthesis of GlyCAM-1, composed of 132 residues. Since then, EPL has been increasingly used to address even more complex biological questions.
EPL for Glycoprotein Synthesis at Creative Biolabs
Powered by advanced platforms for synthesis and analysis, Creative Biolabs has accumulated extensive experience in employing EPL for glycoprotein synthesis. EPL can be performed directly on chitin beads; thiolysis and ligation can occur simultaneously. If an amino acid within or on both ends of the protein sequence is to be glycosylated, the protein would be split in three or more fragments and multi-steps ligation steps would have to be performed. The second peptide fragment, carrying an N-terminal Cys and an additional C-terminal thioester, has to be masked recombinantly at the N-terminus with a protease cleavage site. After the first ligation step, the N-terminal Cys can be free by protease treatment and the second ligation step can be performed. The glycoprotein can be synthesized from the C- to N-direction.
Highlights
-
EPL for glycoprotein synthesis
-
Molecular engineering
-
Powerful chemical glycoprotein synthesis technology
-
Standard analysis techniques
Creative Biolabs has perfected our technical pipelines of EPL for glycoprotein synthesis. If you are interested in our glycoprotein synthesis service, please just feel free to contact us and communicate with us about your specific puzzles.
Reference
-
Muir, T.W. Semisynthesis of proteins by expressed protein ligation. Annual review of biochemistry. 2003, 72(1): 249-89.
For Research Use Only.
Related Services:
- Glycoprotein Remodelling for Glycoprotein Synthesis
- Native Chemical Ligation for Glycoprotein Synthesis
- Staudinger Ligation for Glycoprotein Synthesis
- Sugar-assisted Ligation for Glycoprotein Synthesis
 Fig.1 Recombinant protein α-thioesters and expressed protein ligation. (Muir, 2003)
Fig.1 Recombinant protein α-thioesters and expressed protein ligation. (Muir, 2003)