Glyco-engineered Systems for Therapeutic Glycoprotein Development
Glycosylation serves essential functions on many proteins especially therapeutic antibodies produced in biopharmaceutical manufacturing, making it mandatory to thoroughly consider its biogenesis during the production process. However, protein glycosylation and synthesis of the glycosaminoglycan (GAG) are non-templated, and thus, significant heterogeneity can arise from organism to organism, cell type to cell type, and even between different culture conditions.
A variety of different production systems such as mammalian cells, yeast cells, and plant cells, are currently available for recombinant glycoprotein production purposes. Creative Biolabs has successfully developed our own glyco-engineered systems optimized for improving the productivity of glycoprotein, process robustness, and reducing cell line generation timelines. We offer high-quality customized services by adjusting strategies to meet even the most specific requirements in the production of glycoprotein.
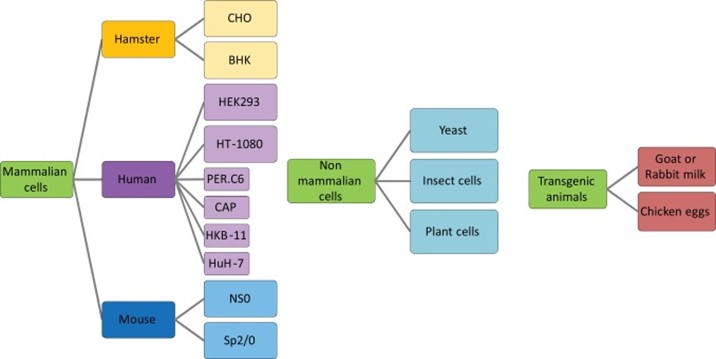 Fig.1 Expression systems used for glycoprotein production by biopharmaceutical industries.1
Fig.1 Expression systems used for glycoprotein production by biopharmaceutical industries.1
Glyco-engineered Systems for Therapeutic Glycoprotein Development at Creative Biolabs
Correct glycan structures are crucial for the pharmacokinetic and pharmacodynamic properties of glycoprotein biologics and therapeutic carbohydrates. Hyperglycosylated proteins show increased serum half-life and are more heat-stable and less sensitive to proteolysis compared with the non-glycosylated forms. Different expression platforms are actively pursued by many academic and industrial laboratories. With years of research and experience in mammalian cell glycoengineering, Creative Biolabs has developed the following glyco-engineered systems for improved glycoprotein production:
-
Glyco-engineered Mammalian Cells
Mammalian cell lines are the preferred host cell expression system to produce proteins with human-like glycosylation patterns. The main cell lines used in this platform include Chinese Hamster Ovary cells (CHO), Human Embryonic Kidney 293 cells (HEK293), Baby Hamster Kidney (BHK21), Human fibrosarcoma (HT1080) and so on. Glycoproteins such as IgG, coagulation factor VII (FVII), erythropoietin (EPO), and alpha-1 antitrypsin (A1AT) could be produced in different mammalian cell lines mentioned above. Four commercial products have been produced in HEK293, B-deleted recombinant FVIII-Fc fusion protein (IgG1), B-deleted recombinant FVIII protein, recombinant FIX-IgG1 Fc fusion protein, and recombinant glucagon-1-like peptide (GLP-1) Fc fusion protein. Except for the above commercial products, many other glycoproteins such as recombinant glycodelin-A (GdA), Iduronate-2-sulfatase, agalsidase alfa, and velaglucerase alfa have also been produced by mammalian cell lines.
Our extensive glycoengineering efforts in both the CHO and HEK 293 systems have substantially improved glycoprotein expression levels and successfully addressed the critical issue of humanizing recombinant glycoproteins.
-
Glyco-engineered Yeast Cells
Yeasts provide high titers recombinant glycoprotein and are easily adapted to fermentation processes in simple and cost-effective culture media. S. cerevisiae and Pichia pastoris have become a substantial workhorse for biotechnology. Using this system, a variety of glycoprotein has been produced with varying degrees of success, such as glucagon, insulin, functional recombinant rabies virus glycoprotein (RABV-G), humanized IgG1, human P-glycoprotein, ovine follicle-stimulating hormone, a Hepatitis B subunit vaccine using Hepatitis B surface antigen; recombinant human granulocyte macrophage-colony stimulating factor, recombinant human platelet-derived growth factor, human papillomavirus subunit vaccine, a kallikrein inhibitor, human P-glycoprotein, ovine follicle-stimulating hormone and so on.
We've reconfigured the glycosylation pathway of Pichia pastoris and effectively established a glycoengineered Pichia pastoris expression system. This system offers notable benefits, such as a reduced production timeline, cost-efficiency, high yield, simplicity of operation, and scalability.
-
Glyco-engineered Plant Cells
Plant cells can be easily cultured in basal culture medium and present a robust cell growth, easily scaled up. Additionally, they do not harbor human-trophic pathogens and endotoxins and are not subject to several disadvantages of recombinant glycoprotein produced in whole plants. Elelyso which is intended for the treatment of Gaucher's disease was the first recombinant glycoprotein produced in an engineered carrot plant root cell line that hit the market in 2012. Alpha galactosidase-A, human tumor necrosis factor receptor II, glucocerebrosidase, interferon alpha, and even a humanized and glyco-optimized anti-CD20 antibody have also been acquired through plant expression systems.
By eliminating nonhuman glycans and introducing mammalian enzymes for the generation of humanized glycans, we have developed an efficient glyco-engineered plant-based expression system. This system is adaptable to various glycan requirements with different therapeutic needs.
Features of Our Glyco-engineered Systems
-
Optional and efficient expression systems
-
Advanced and patented glycoengineering technology
-
Flexible glycosylation controls
-
Enhanced biological activity of therapeutic glycoproteins
-
High-yield expression level of recombinant glycoprotein.
-
Short production period, scalability, and improved safety.
Creative Biolabs is committed to providing optional glyco-engineered expression systems for therapeutic glycoprotein development and high-quality glycoprotein products to promote the progress of your projects. Please feel free to contact us for more information and a detailed quote.
Reference
-
Lalonde, Marie-Eve, and Yves Durocher. "Therapeutic glycoprotein production in mammalian cells." Journal of Biotechnology 251 (2017): 128-140.
For Research Use Only.
 Fig.1 Expression systems used for glycoprotein production by biopharmaceutical industries.1
Fig.1 Expression systems used for glycoprotein production by biopharmaceutical industries.1